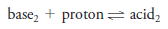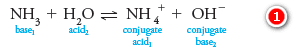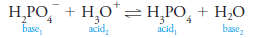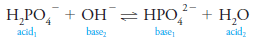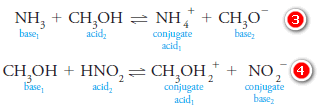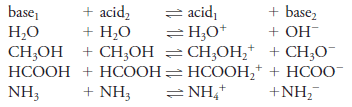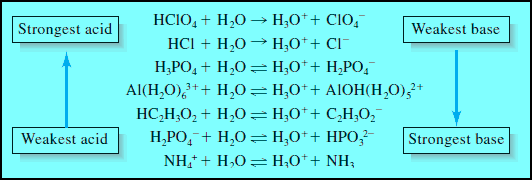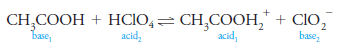The Chemical Composition of Aqueous Solution
– In this subject, we will discuss the Chemical Composition of Aqueous Solution
– Water is the most plentiful solvent on Earth, is easily purified, and is not toxic.
– It is, therefore, widely used as a medium for chemical analyses.
Classifying Solutions of Electrolytes
– Most of the solutes we will discuss are electrolytes, which form ions when dissolved in water (or certain other solvents) and thus produce solutions that conduct electricity.
– Strong electrolytes ionize essentially completely in a solvent, but weak electrolytes ionize only partially.
– These characteristics mean that a solution of a weak electrolyte will not conduct electricity as well as a solution containing an equal concentration of a strong electrolyte.
– Table (1) shows various solutes that act as strong and weak electrolytes in water.
– Among the strong electrolytes listed are acids, bases, and salts.
– *H2SO4 is completely dissociated into HSO4– and H3O+ions and for this reason is classified as a strong electrolyte. Note, however, that the HSO4+ ion is a weak electrolyte and is only partially dissociated into SO42- and H3O+
Acids and Bases
– In 1923, J. N. Brønsted in Denmark and J. M. Lowry in England proposed independently a theory of acid/base behavior that is especially useful in analytical chemistry.
– According to the Brønsted-Lowry theory, an acid is a proton donor, and a base is a proton acceptor.
– For a molecule to behave as an acid, it must encounter a proton acceptor (or base).
– Likewise, a molecule that can accept a proton behaves as a base if it encounters an acid.
Conjugate Acids and Bases
– An important feature of the Brønsted-Lowry concept is the idea that the product formed when an acid gives up a proton is a potential proton acceptor and is called the conjugate base of the parent acid.
– For example, when the species acid1 gives up a proton, the species base1 is formed, as shown by the reaction:
– We refer to acid1 and base1 as a conjugate acid/base pair, or just a conjugate pair. Similarly, every base accepts a proton to produce a conjugate acid. That is,
– When these two processes are combined, the result is an acid/base, or neutralization, reaction:
– This reaction proceeds to an extent that depends on the relative tendencies of the two bases to accept a proton (or the two acids to donate a proton).
– Examples of conjugate acid/base relationships are shown in Equations (1) through (4).
– Many solvents are proton donors or proton acceptors and can thus induce basic or acidic behavior in solutes dissolved in them.
– For example, in an aqueous solution of ammonia, water can donate a proton and act as an acid with respect to the solute NH3:
– In this reaction, ammonia (base1) reacts with water, which is labeled acid2, to give the conjugate acid ammonium ion (acid1) and hydroxide ion, which is the conjugate base (base2) of the acid water.
– On the other hand, water acts as a proton acceptor, or base, in an aqueous solution of nitrous acid:
– The conjugate base of the acid HNO2 is a nitrite ion. The conjugate acid of water is the hydrated proton written as H3O+.
– This species is called the hydronium ion, and it consists of a proton covalently bonded to a single water molecule.
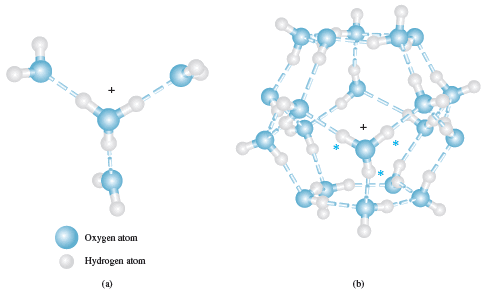
– Higher hydrates such as H5O2+, H9O4+, and the dodecahedral cage structure shown in Figure (1) may also appear in aqueous solutions of protons.
– For convenience, however, we generally use the notation H3O+, or more simply H+, when we write chemical equations containing the hydrated proton.
– Figure (1) Possible structures for the hydronium ion.
(a) The species H9O4+ has been observed in the solid state and may be an important contributor to aqueous solution.
(b) The species (H2O)20H+ exhibits a dodecahedral caged structure.
– The extra proton in the structure, which may be any one of the three marked with an asterisk, is free to move around the surface of the dodecahedron by being transferred to an adjacent water molecule
– An acid that has donated a proton becomes a conjugate base capable of accepting a proton to reform the original acid.
– Similarly, a base that has accepted a proton becomes a conjugate acid that can donate a proton to form the original base.
– Thus, nitrite ion, the species produced by the loss of a proton from nitrous acid, is a potential acceptor of a proton from a suitable donor.
– It is this reaction that causes an aqueous solution of sodium nitrite to be slightly basic:

Notes on acids and bases
(1) A salt: is produced in the reaction of an acid with a base. Examples include NaCl, Na2SO4, and NaOOCCH3 (sodium acetate).
(2) An acid: donates protons. A base: accepts protons.
(3) An acid donates protons only in the presence of a proton acceptor (a base). Likewise, a base accepts protons only in the presence of a proton donor (an acid).
(4) A conjugate base: is formed when an acid loses a proton. For example, acetate ion is the conjugate base of acetic acid. Similarly, ammonium ion is the conjugate acid of the base ammonia.
(5) A conjugate acid: is formed when a base accepts a proton.
(6) A substance acts as an acid only in the presence of a base and vice versa.
Amphiprotic Species
– Species that have both acidic and basic properties are amphiprotic.
– An example is the dihydrogen phosphate ion, H2PO4–, which behaves as a base in the presence of a proton donor such as H3O+
– Here, H3PO4 is the conjugate acid of the original base.
– In the presence of a proton acceptor, such as hydroxide ion, however, H2PO4 – behaves as an acid and donates a proton to form the conjugate base HPO42-
– The simple amino acids are an important class of amphiprotic compounds that contain both a weak acid and a weak base functional group.
– When dissolved in water, an amino acid, such as glycine, undergoes a kind of internal acid/base reaction to produce a zwitterion—a species that has both a positive and a negative charge.
– Thus,
– This reaction is analogous to the acid/base reaction between a carboxylic acid and an amine:
– Water is the classic example of an amphiprotic solvent, that is, a solvent that can act either as an acid (Equation 1) or as a base (Equation 2), depending on the solute.
– Other common amphiprotic solvents are methanol, ethanol, and anhydrous acetic acid.
– In methanol, for example, the equilibria analogous to the water equilibria shown in Equations (1) and (2) are:
Notes:
– A zwitterion is an ion that has both a positive and a negative charge.
– Water can act as either an acid or a base.
– Amphiprotic solvents behave as acids in the presence of basic solutes and bases in the presence of acidic solutes.
Autoprotolysis
– Amphiprotic solvents undergo self-ionization, or autoprotolysis, to form a pair of ionic species.
– Autoprotolysis is yet another example of acid/base behavior, as illustrated by the following equations:
– The extent to which water undergoes autoprotolysis at room temperature is slight.
– Thus, the hydronium and hydroxide ion concentrations in pure water are only about 10-7 M.
– Despite the small values of these concentrations, this dissociation reaction is of utmost importance in understanding the behavior of aqueous solutions.
Note: Autoprotolysis (also called autoionization) is the spontaneous reaction of molecules of a substance to give a pair of ions.
Strengths of Acids and Bases
– Figure (2) shows the dissociation reactions of a few common acids in water.
(a) The first two are strong acids because the reaction with the solvent is sufficiently complete that no undissociated solute molecules are left in the aqueous solution.
(b) The rest are weak acids, which react incompletely with water to give solutions containing significant quantities of both the parent acid and its conjugate base.
– Note that acids can be cationic, anionic, or electrically neutral. The same holds for bases.
– The acids in Figure (2) become progressively weaker from top to bottom.
– Perchloric acid and hydrochloric acid are completely dissociated, but only about 1% of acetic acid (HC2H3O2) is dissociated.
– Ammonium ion is an even weaker acid with only about 0.01% of this ion being dissociated into hydronium ions and ammonia molecules.
– Another generality illustrated in Figure (2) is that the weakest acid forms the strongest conjugate base, that is, ammonia has a much stronger affinity for protons than any base above it.
– Perchlorate and chloride ions have no affinity for protons.
– The tendency of a solvent to accept or donate protons determines the strength of a solute acid or base dissolved in it.
For example, perchloric and hydrochloric acids are strong acids in water.
– If anhydrous acetic acid, a weaker proton acceptor than water is substituted as the solvent, neither of these acids undergoes complete dissociation.
– Instead, equilibria such as the following are established:
– Perchloric acid is, however, about 5000 times stronger than hydrochloric acid in this solvent.
– Acetic acid thus acts as a differentiating solvent toward the two acids by revealing the inherent differences in their acidities.
– Water, on the other hand, is a leveling solvent for perchloric, hydrochloric, and nitric acids because all three are completely ionized in this solvent and show no differences in strength.
– There are differentiating and leveling solvents for bases as well.
– The common strong bases include NaOH, KOH, Ba(OH)2, and the quaternary ammonium hydroxide R4NOH, where R is an alkyl group such as CH3 or C2H5.
– The common strong acids include HCl, HBr, HI, HClO4, HNO3, the first proton in H2SO4, and the organic sulfonic acid RSO3H.
– Reference: Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition), 2014 . USA