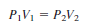Boyle’s Law: Definition, Mathematical, Graphical, Applications
– In this subject, we will discuss Boyle’s Law: Definition, Mathematical, Graphical, Applications
How did Boyle discover his Law?
– Robert Boyle (1627–1691) is a British chemist and natural philosopher.
– Although Boyle is commonly associated with the gas law that bears his name, he made many other significant contributions to chemistry and physics.
– Although Boyle was often at odds with scientists of his generation, his book The Skeptical Chemist (1661) influenced generations of chemists.
– In the seventeenth century, Robert Boyle studied the behavior of gases systematically and quantitatively.
– In one series of studies, Boyle investigated the pressure-volume relationship of a gas sample.
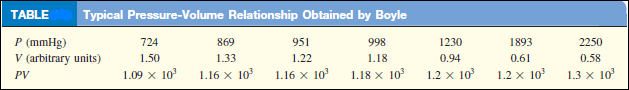
– Typical data collected by Boyle are shown in the following Table:
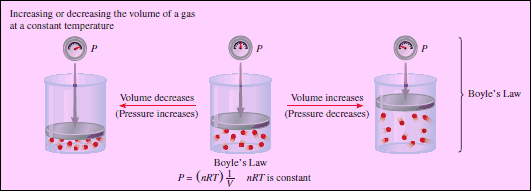
– Boyle noted that as the pressure (P) is increased at a constant temperature, the volume (V) occupied by a given amount of gas decreases.
– Compare the first data point with a pressure of 724 mmHg and a volume of 1.50 (in arbitrary unit) to the last data point with a pressure of 2250 mmHg and a volume of 0.58.
– Clearly, there is an inverse relationship between the pressure and volume of a gas at a constant temperature.
– As the pressure is increased, the volume occupied by the gas decreases.
– Conversely, if the applied pressure is decreased, the volume the gas occupies increases.
– This relationship is now known as Boyle’s law.
Definition of Boyle’s Law
– Boyle’s law states that:
” The pressure of a fixed amount of gas at a constant temperature is inversely proportional to the volume of the gas “.
– The apparatus used by Boyle in this experiment was very simple. The temperature and amount of gas are kept constant.
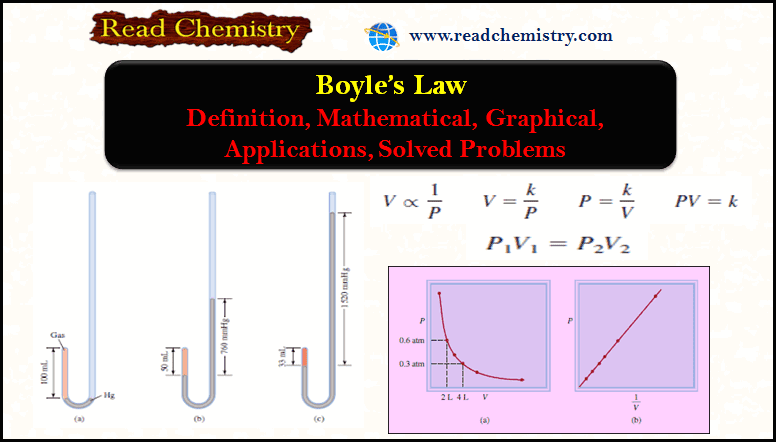
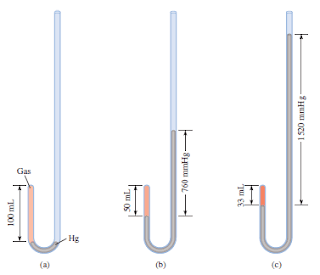
In Figure (a):
– the pressure exerted on the gas is equal to atmospheric pressure and the volume of the gas is 100 mL.
– (Note that the tube is open at the top and is therefore exposed to atmospheric pressure.)
In Figure (b):
– More mercury has been added to double the pressure on the gas, and the gas volume decreases to 50 mL.
In Figure (c):
– Tripling the pressure on the gas decreases its volume to a third of the original value.
Mathematical Expression of Boyle’s Law
– We can write a mathematical expression showing the inverse relationship between pressure and volume:
– where the symbol α means proportional to.
– We can change α to an equals sign and write
– where k1 is a constant called the proportionality constant.
– Equation (1) is the mathematical expression of Boyle’s law.
– We can rearrange Equation (1) and obtain:
– This form of Boyle’s law says that the product of the pressure and volume of a gas at constant temperature and amount of gas is constant.
– The following diagram is a schematic representation of Boyle’s law.
– The quantity n is the number of moles of the gas and R is a constant.
Graphical Representation of Boyle’s Law
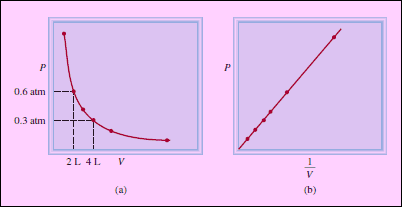
Figure (a): is a graph of the equation: PV = k1
Figure (b): is a graph of the equivalent equation: P = k1 × 1/V.
– Note that the latter is a linear equation of the form y = mx + b, where b = 0 and m = k1.
– Although the individual values of pressure and volume can vary greatly for a given sample of gas, as long as the temperature is held constant and the amount of the gas does not change, P times V is always equal to the same constant.
– Therefore, for a given sample of gas under two different sets of conditions at constant temperature, we have:

– where V1 and V2are the volumes at pressures P1 and P2 , respectively
Importance of Boyle’s law
Human breathing system
– Boyle’s law is often used as part of an explanation of how the breathing system works in the human body.
– This commonly involves explaining how the lung volume may be increased or decreased and thereby cause a relatively lower or higher air pressure within them (in keeping with Boyle’s law).
– This forms a pressure difference between the air inside the lungs and the environmental air pressure, which in turn precipitates either inhalation or exhalation as air moves from high to low pressure
Applications of Boyle’s Law
- Spray paint
- The syringe
- The soda can
- The bends
(1) Spray Paint
– While there are a couple of different types of aerosol cans, some being a little more elaborate than others, they all rely on the same basic principle: Boyle’s law.
– Before you spray a can of paint, you are supposed to shake it up for a while as a ball bearing rattles around inside.
– There are two substances inside the can: one is your product (paint for example), and the other is a gas that can be pressurized so much that it retains a liquid state, even when it is heated past its boiling point.
– This liquefied gas has a boiling point far below room temperature. Because the can is sealed, the gas is prevented from boiling and turning into a gas. That is until you push down the nozzle.
– The moment the nozzle of a spray paint can go down, the seal is broken and the propellant instantly boils, expands into a gas, and pushes down on the paint.
– Under high pressure, the paint is forced out of the nozzle as it attempts to reach an area with lower pressure.
(2) The Syringe
– This mechanism is far more simple than a can of spray paint. Syringes of all types utilize Boyle’s law on a very basic level.
– When you pull the plunger out on a syringe, it causes the volume within the chamber to increase.
– As we know, this causes the pressure to do the opposite, which then creates a vacuum.
– When a syringe is empty, the vacuum within the chamber sucks fluid in through the needle.
(3) The Soda Can or Bottle
– Typically when we open a bottle of soda, we slowly turn the cap to allow the air to escape before we completely remove the lid.
– We do this because we’ve learned over time that twisting it open too fast causes it to fizz up and spill all over.
– This happens because the liquid is pumped full of carbon dioxide, causing it to bubble up as the CO2 makes its escape.
– When a soda bottle is filled, it is also pressurized.
– Much like the aerosol can mentioned earlier when you slowly open the cap, the gas can increase its volume and the pressure decreases.
– Normally you can let the gas out of a can or bottle release cleanly, but if the bottle is shaken up and the gas is mixed into the liquid, then you may have a mess on your hands.
– This is because the gas trying to escape is mixed into the fluid, so, when it does escape, it brings the foamy fluid out with it.
– Pressure in the bottle goes down, the volume of the gas goes up, and you have yourself a mess to clean up.
(4) The Bends
– Any properly trained scuba diver knows when they are ascending from deep waters, a slow ascension is critical.
– Our bodies are built for and accustomed to living in the normal pressure of our lower atmosphere.
– As a diver goes deeper underwater, that pressure begins to increase.
– Water is heavy, after all. With the increasing pressure causing a decrease in volume, nitrogen gasses begin to be absorbed by the diver’s blood.
– When the diver begins his ascent and the pressure is lessened, these gas molecules begin to expand back to their normal volume.
– With a slow ascent, or through the use of a depressurization chamber, those gasses can work their way back out of the bloodstream slowly and normally.
– But if the diver ascends too quickly, the blood in their veins becomes a foamy mess.
– The same thing that happens to a foamy soda is what happens to a diver’s bloodstream during the bends.
– On top of that, any built-up nitrogen between the diver’s joints will also expand, causing the diver to bend over (hence its name) in severe pain.
– In the worst cases, this sudden depressurization of the body can kill a person instantly.
Solved Problems on Boyle’s Law
Example (1)
The following data were obtained in a laboratory experiment:
(a) Plot these data, and determine what pressure is required to get a volume of 0.600 L.
(b) Determine the pressure required to get a volume of 0.150 L.
(c) Plot P versus 1/V, and determine the two pressures again.
Solution:
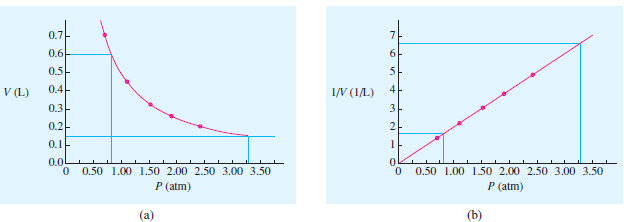
(a) The plot of the data is shown in Figure (a). With the pressure is read as 0.82 atm.
(b) In Figure (a), the pressure is estimated to be 3.3 atm.
(c) The plot is shown in Figure (b). With V = 0.600 L, 1/V = 1.67/L, and the pressure is read as 0.82 atm.
– With V = 0.150 L, 1/V = 6.67/L. and the pressure is 3.26 atm.
– The linear plot of Figure (b) makes it easy to estimate data beyond the experimental points.
– Estimations beyond the experimental points are not as easy on the curve of Figure (a) because how much the curve bends is difficult to predict.
Example (2)
A 3.50-L sample of gas has a pressure of 0.750 atm. Calculate the volume after its pressure is increased to 1.50 atm at a constant temperature.
Solution:
In this type of problem, tabulating the given data is useful
Substitution of the values into the equation yields
Note that multiplying the pressure by 2 causes the volume to be reduced to half.
Example (3)
A 1.45-L sample of gas has a pressure of 0.950 atm. Calculate the volume after its pressure is increased to 787 torr at constant temperature.
Solution:
– Because the pressures are given in two different units, one of them must be changed.
– Now the problem can be solved as in Example 1:
– Alternatively, we could change the 0.950 atm to torr:
Example (4)
Calculate the pressure required to change a 3.38-L sample of gas initially at 1.15 atm to 925 mL, at constant temperature.
Solution:
– The pressure must be raised to 4.20 atm.
Reference:
- Chemistry / Raymond Chang, Williams College /(10th edition).
- Fundamentals of Chemistry / David E.Goldberg/(5th edition).