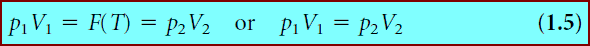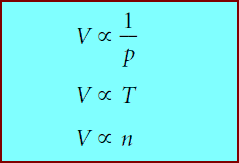** Phenomenological thermodynamics is based on experiment, on measurements that you might make in a lab, garage, or kitchen.
** For example, for any fixed amount of a pure gas, two state variables are pressure, p, and volume, V. Each can be controlled independently of each other.
** The pressure can be varied while the volume is kept constant, or vice versa.
** Temperature, T, is another state variable that can be changed independently from p and V.
** However, experience has shown that if a certain pressure, volume, and temperature were specified for a particular sample of gas at equilibrium, then all measurable, macroscopic properties of that sample have certain specific values.
** That is, these three state variables determine the complete state of our gas sample. Notice that we are implying the existence of one other state variable: amount.
** The amount of material in the system, designated by n, is usually given in units of moles.
** Further, arbitrary values for all four variables p, V, n, and T are not possible simultaneously. Again, experience (that is, experiment) shows this.
** It turns out that only two of the three state variables p, V, and T are truly independent for any given amount of a gas. Once two values are specified, then the third one must have a certain value. This means that there is a mathematical equation into which we can substitute for two of the variables and calculate what the remaining variable must be.
** Say such an equation requires that we know p and V and lets us calculate T. Mathematically, there exists some function F such that:
where the function is written as F(p, V) to emphasize that the variables are pressure and volume, and that the outcome yields the value of the temperature T.
** Equations like equation 1.1 are called equations of state.
** One can also define equations of state that yield p or V instead of T. In fact, many equations of state can be algebraically rearranged to yield one of several possible state variables.
Ideal gas law
** The earliest equations of state for gases were determined by Boyle, Charles, Amontons, Avogadro, Gay-Lussac, and others.We know these equations as the various gas laws.
(1) In the case of Boyle’s gas law, the equation of state involves multiplying the pressure by the volume to get a number whose value depended on the temperature of the gas:
(2) whereas Charles’s gas law involves volume and temperature:
(3) Avogadro’s law relates volume and amount, but at fixed temperature and pressure:
** In the above three equations, if the temperature, pressure, or amount were kept constant, then the respective functions F(T), F(p), and F(n) would be constants. This means that if one of the state variables that can change does, the other must also change in order for the gas law to yield the same constant. This leads to the familiar predictive ability of the above gas laws using the forms
** Similarly, using equations 1.3 and 1.4, we can get:
** All three gas laws involve volume, and they can be rewritten as:
** where the symbol α means “is proportional to.’’We can combine the three proportionalities above into one:
** Since p, V, T, and n are the only four independent state variables for a gas, the proportionality form of equation 1.8 can be turned into an equality by using a proportionality constant:
** where we use R to represent the proportionality constant. This equation of state relates the static (unchanging) values of p, V, T, and n, not changes in these values. It is usually rewritten as:
** which is the familiar ideal gas law, with R being the ideal gas law constant.
Fahrenheit and Celsius temperature
** At this point, we must return to a discussion of temperature units and introduce the proper thermodynamic temperature scale.
** It has already been mentioned that the Fahrenheit and Celsius temperature scales have arbitrary zero points.
** What is needed is a temperature scale that has an absolute zero point that is physically relevant.Values for temperature can then be scaled from that point.
** In 1848, the British scientist William Thomson, later made a baron and taking the title Lord Kelvin, considered the temperature-volume relationship of gases and other concerns (some of which we will address in future chapters) and proposed an absolute temperature scale where the minimum possible temperature is about -273°C, or 273 Celsius-sized degrees below the freezing point of water. [A modern value is -273.15°C, and is based on the triple point of H2O, not the freezing point.]. A scale was established by making the degree size for this absolute scale the same as the Celsius scale.
** In thermodynamics, gas temperatures are almost always expressed in this new scale, called the absolute scale or the Kelvin scale, and the letter K is used (without a degree sign) to indicate a temperature in kelvins.
** Because the degree sizes are the same, there is a simple conversion between a temperature in degrees Celsius and the same temperature in kelvins:
** Occasionally, the conversion is truncated to three significant figures and becomes simply K= °C + 273.
** In all of the gas laws given above, the temperature must be expressed in kelvins! The absolute temperature scale is the only appropriate scale for thermodynamic temperatures. (For changes in temperature, the units can be kelvins or degrees Celsius, since the change in temperature will be the same. However, the absolute value of the temperature will be different.)
The ideal gas law constant
** Having established the proper temperature scale for thermodynamics, we can return to the constant R.
** This value, the ideal gas law constant, is probably the most important physical constant for macroscopic systems.
** Specific numerical value of R depends on the units used to express the pressure and volume, since the units in an equation must also satisfy certain algebraic necessities.
** Table shows lists various values of R.
Notes
** The ideal gas law is the best known equation of state for a gaseous system.
** Gas systems whose state variables p, V, n, and T vary according to the ideal gas law satisfy one criterion of an ideal gas .
** Real gases, which do not follow the ideal gas law exactly, can approximate ideal gases if they are kept at high temperature and low pressure.
Standard temperature and pressure (STP)
** It is useful to define a set of reference state variables for gases, since they can have a wide range of values that can in turn affect other state variables.
** The most common set of reference state variables for pressure and temperature is p = 1.0 atm and T = 273.15 K = 0.0°C.
** These conditions are called standard temperature and pressure, abbreviated STP. Much of the thermodynamic data reported for gases are given for conditions of STP.
** SI also defines standard ambient temperature and pressure, SATP, as 273.15 K for temperature and 1 bar for pressure (1 bar = 0.987 atm).
Example: Calculate the volume of 1 mole of an ideal gas at SATP.
Solution:
Using the ideal gas law and the appropriate value for R:
This is slightly larger than the commonly used molar volume of a gas at STP (about 22.4 L), since the pressure is slightly lower.
Reference: physical Chemistry /David W. Ball / Cleveland State University /2011 .
 Read Chemistry
Read Chemistry