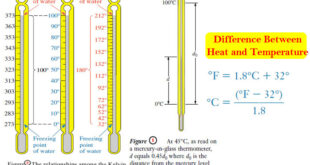
– In this subject, we will discuss Acid-Base Equilibrium in Water.
Acid-Base Equilibrium in Water
– When an acid or base is dissolved in water, it will dissociate, or ionize According to the equations:
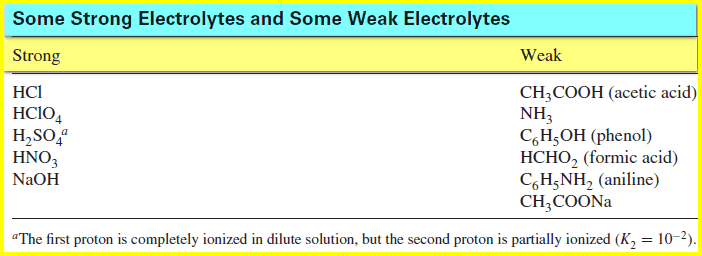
– The amount of ionization is dependent on the strength of the acid or the base. A “strong” electrolyte is completely dissociated, while a “weak” electrolyte is partially dissociated.
– The table below lists some common electrolytes, some strong and some weak:
– Hydrochloric acid is a strong acid, and in water, its ionization is complete:

– An equilibrium constant for the Equation above would have a value of infinity.
– The proton H+ exists in water as a hydrated ion, the hydronium ion, H3O+.
– Higher hydrates probably exist, particularly H9O4+.
– The hydronium ion is written as H3O+ for convenience and to emphasize Brønsted behavior.
– Acetic acid is a weak acid, which ionizes only partially in water (a few percent):
– We can write an equilibrium constant for this reaction:

Where:
K◦a = the thermodynamic acidity constant
a = the activity of the indicated species.
– Salt cations or anions may also partially react with water after they are dissociated.
– For example, acetate ion is formed from dissociated acetate salts, to give HOAc.
– The activity can be thought of as representing the effective concentration of an ion.
– The effects of protons in reactions are often governed by their activities, and it is the activity that is measured by the widely used pH meter.
– In dilute solutions, the activity of water remains essentially constant and is taken as unity at the standard state. Therefore, Equation can be written as:
– Pure water ionizes slightly, or undergoes autoprotolysis (Autoprotolysis is the self-ionization of a solvent to give a characteristic cation and anion)
– The equilibrium constant for this is:
– Again, the activity of water is constant in dilute solutions (its concentration is essentially constant at ∼55.5 M), so:
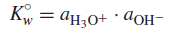
where K◦w = the thermodynamic autoprotolysis, or self-ionization, constant
– Calculations are simplified if we neglect activity coefficients.
– This simplification results in only slight errors for dilute solutions, and we shall use molar concentrations in all our calculations.
– This will satisfactorily illustrate the equilibria involved.
– Most of the solutions we will be concerned with are rather dilute, and we will frequently be interested in relative changes in pH (and large ones) in which case small errors are insignificant.
– We will simplify our expressions by using H+ in place of H3O+.
– This is not inconsistent since the waters of solvation associated with other ions or molecules (e.g., metal ions) are not generally written and H3O+ is not an accurate representation of the actual species present; typically the proton in dilute aqueous solution has at least four water molecules in its solvation shell.
Conclusion
– Molar concentration will be represented by square brackets [ ] around the species.
– Simplified equations for the above reactions are:
Ka and Kw are the molar equilibrium constants.
– Kw is exactly 1.00 × 10−14 at 24◦C and even at 25◦C, to a smaller number of significant figures, it is still accurately represented as 1.0 × 10−14.
– The product of the hydrogen ion concentration and the hydroxide ion concentration in aqueous solution is always equal to 1.0 × 10−14 at room temperature:
– In pure water, then, the concentrations of these two species are equal since there are no other sources of H+ or OH− except H2O dissociation:

Therefore
– If an acid is added to water, we can calculate the hydroxide ion concentration if we know the hydrogen ion concentration from the acid.
– But when the hydrogen ion concentration from the acid is very small, 10−6 M or less, the contribution to [H+] from the ionization of water cannot be neglected.
Solved Problem on Acid-Base Equilibrium in Water
Example: A 1.0 × 10−3 M solution of hydrochloric acid is prepared. What is the hydroxide ion concentration?
Solution:
– Since hydrochloric acid is a strong electrolyte and is completely ionized, the H+ concentration is 1.0 × 10−3 M.
– Thus,
Reference: Analytical chemistry/ Seventh edition / Gary D. Christian, University of Washington, Purnendu K. (Sandy) Dasgupta, University of Texas at Arlington, Kevin A. Schug, University of Texas at Arlington.
 Read Chemistry
Read Chemistry



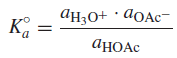

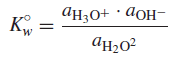
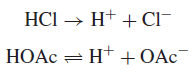
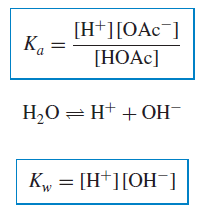
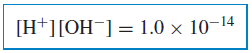
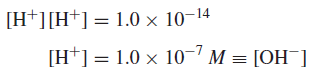
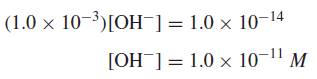


https://cse.google.co.jp/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.fr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.es/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.es/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.it/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.hk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.nl/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.in/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ru/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.pl/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.tw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.id/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.cz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.se/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.hu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.ar/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.ar/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.fi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.nz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.pt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ro/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.ro/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.gr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.ph/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.ph/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.no/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.no/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.no/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.il/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ie/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.sk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.kr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.co.kr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.co.kr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.pe/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.eg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.lt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.hr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.rs/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.rs/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.si/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.by/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.lv/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.ba/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ba/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.ng/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.ng/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.ng/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.cf/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.cf/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.pr/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.gt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.uy/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.uy/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.lu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.kw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.kw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.is/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.is/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.dz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.dz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.ke/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.tn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.tn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.tn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.az/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.az/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.am/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.np/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.np/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.iq/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.iq/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.iq/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.to/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.cm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.cm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.bd/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.bd/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.kz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.kz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.kz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.ma/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.jo/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.jo/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ge/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.ge/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.ge/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.lk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.lk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.ni/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.ni/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.md/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.mg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.mg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.mg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.la/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.la/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.la/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.jm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.jm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.jm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.cy/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.cy/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.qa/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.qa/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.sv/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.sv/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.rw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.rw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ps/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.ps/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.bo/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.bo/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.bs/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.bs/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.mu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.mu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.mu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.mk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.mk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.mk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.al/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.al/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.li/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.li/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.li/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.mn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.mn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.mn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.bh/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.bh/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.kh/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.kh/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.lb/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.lb/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.tt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.tt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ci/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.ci/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.ci/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.dj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.dj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.hn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.hn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.co.tz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.tz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.gm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.gm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.sn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.sn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.st/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.mt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.mt/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.cat/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.bi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.bi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.bi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.gg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.gg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.ly/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.ly/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.ly/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.na/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.na/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.me/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.cd/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.cd/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.cd/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.fm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.fm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.fm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.as/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.as/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.com.et/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.et/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.et/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.pa/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.ht/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ht/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.ai/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.gi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.gi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.gi/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.vu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.vu/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.vg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.vg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.vg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.im/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.im/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.cf/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.pn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.pn/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://www.google.tg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.tg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.vc/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.tj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.com.om/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.om/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.tk/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.co.mz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.mz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.co.mz/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.co.bw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ne/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.sm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.sm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.zw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.co.zw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.co.zw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.tm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.tm/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.gy/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.af/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.fj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.com.fj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.com.sl/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.cg/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.ki/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.mw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.mw/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.bj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.bj/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.st/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.td/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://cse.google.co.ao/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://maps.google.co.ao/url?q=https%3A%2F%2Fwww.learnchemistry13.com
https://images.google.co.ao/url?q=https%3A%2F%2Fwww.learnchemistry13.com