Electron Configuration Of Elements
– In this subject, we will discuss the rules of Electron Configuration Of Elements
Electron Configuration Of Elements
– We have seen before that to define completely the state of an atom it is obligatory to refer to all the four quantum numbers (n, l, m, and s) of every electron in it.
– Since a simultaneous representation of all quantum numbers of each electron in a single symbolic notation seems quite difficult, it is customary to take into account the first two quantum numbers only while the other two can be inferred indirectly.
– The general symbolic notation employed for the purpose is: nl a
where:
– The numerical value of n = 1, 2, 3 etc., represents the principal quantum number,
– The letter designate of (l) (s for l = 0, p for l= 1 and so on) stands for the orbital
– and The superscript (a) gives the number of electrons in the orbital.
Example:
3s2 indicates that two electrons are present in the first subshell s (l = 0) of the third shell (n = 3).
– For instance, the distribution of seven electrons (of N atom) may be schematically represented as: 1s2; 2s2, 2p3or more elaborately as: 1s1; 2s2, 2px1, 2py1 , 2pz1 By using the various designates of orbitals at a sublevel such as 2px , 2py etc.,
– The third quantum number (m) is also indicated (e.g., 2px, for m = + 1, 2py for m = 0 and 2pz for m= – 1).
– Spin quantum numbers are indirectly inferred.
– Whenever there are two electrons in an orbital, one of these has +½ and the other −½ as their spin quantum number.
How to present the Orbital and electron
(1) It is a common practice to denote an orbital by a horizontal line or a circle or square and an electron by an arrow over it.
(2) The direction of the arrow indicates the spin, an upward arrow representing a clockwise spin while the downward arrow stands for the anticlockwise direction of spin.
(3) When there are more than one orbitals in a subshell (degenerate orbitals), they are shown by an equivalent number of horizontal lines at the same energy level.
Electron configuration of first ten elements.
Let us now describe the electron configuration of the first ten elements.
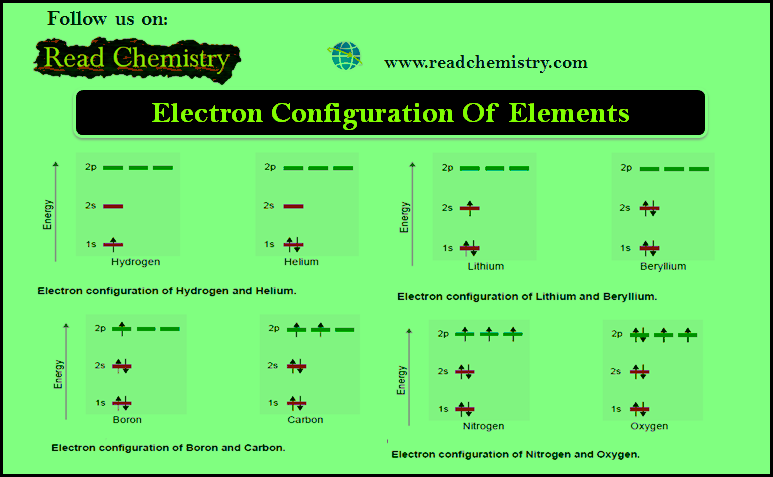
(a) Hydrogen and Helium
– These have one and two electrons respectively which are accommodated in 1s orbital while others remain vacant.
– The lone electron of hydrogen is filled in 1s orbital and for helium the second electron would also go in 1s orbital, since it could accommodate another electron with opposite spin.
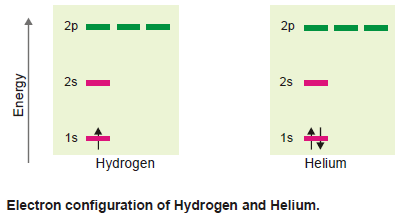
(b) Lithium and Beryllium
– These have three and four electrons respectively.
– The third electron of Li enters in the 2s orbital and the fourth electron of Be also enters in the same orbital, but has an opposite direction of spin.
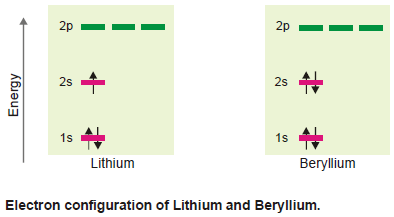
(c) Boron and Carbon
– These atoms have five and six electrons respectively.
– 1s and 2s orbitals being completely filled with four electrons, the fifth electron of boron would go in one of the 2p orbitals say 2px.
– The sixth electron in carbon would prefer to be accommodated in another vacant 2p orbital say (2py) rather than going to 2pz orbital (Hund’s rule).
– The two unpaired electrons shall have similar spins as indicated.
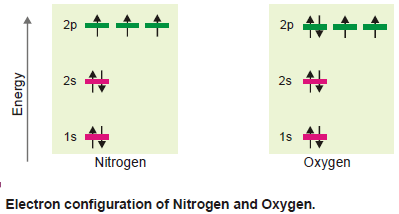
(d) Nitrogen and Oxygen
– These atoms have seven and eight electrons respectively.
– After six electrons have been accommodated as above, there is a vacant 2pz orbital which will be the seat of the seventh electron possessing the same direction of spin.
– The eighth electron of the next element oxygen will go to pair up with the 2px electron and has an antiparallel spin as shown below.
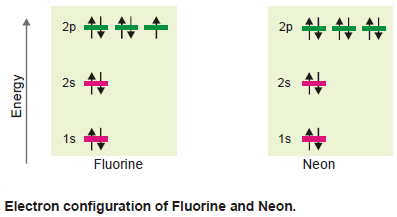
(e) Fluorine and Neon
– These atoms possess nine and ten electrons respectively which go to complete the other 2p orbitals as shown in the following Figure:
The ground-state electron configuration of elements
– So far we have considered the electron configuration of simple atoms.
– For complicated atoms which may contain many electrons and have many energy levels or orbitals, the ‘building up’ process for the electrons is governed by the following rules :
Rule (1)
Each electron shell can hold a maximum of 2n2 electrons where (n) is the shell number.
Rule (2)
These electrons are accommodated in s, p, d, and f orbitals, the maximum number of electrons in each type of orbitals being determined by its electron-holding capacity (for s = 2, p = 6, d = 10, and f = 14).
Rule (3)
In the ground state of an atom, the electrons tend to occupy the available orbitals in the increasing order of energies, the orbitals of lower energy being filled first.
– This is called building up principle or Aufbau Principle (Aufbau is a German expression meaning building up or construction).
– Lower energy orbitals are, therefore, better seats for electrons, and better seats are occupied first.
– The Following figure shows the energy level scheme of orbitals and this order can conveniently be remembered by the simple device given below.
– The increasing order of energy of various orbitals is as follows:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s ……
– The energy of an orbital is determined by the sum of principal quantum number (n) and the azimuthal quantum number (l). This rule is called (n + l) rule.
– There are two parts of this rule:
(a) The orbitals with the lower value of (n + l) has lower energy than the orbitals of higher (n + l) value.
(b) When two orbitals have same (n + l) value, the orbital with lower value of n has lower energy.
– For example: let us compare the (n + l) value for 3d and 4s orbitals:
– For 3d orbital n = 3, l = 2 and n + l = 5 and for 4s orbital n = 4, l = 0 and n + l = 4.
– Therefore, 4s orbital is filled before 3d orbital. Similarly, for 4p and 5s orbitals, the (n + l) values are (4 + 1) and (5 + 0) respectively.
– In this case 4p orbital has lesser value of (n) and hence it has lower energy than 5d orbital and is filled first.
– It is, therefore, clear from above that 4s orbital would be filled before 3d orbitals (belonging to a lower shell i.e., third) are filled because the latter have higher energy than the former.
Rule (4)
Any orbital may have one or two electrons at the most.
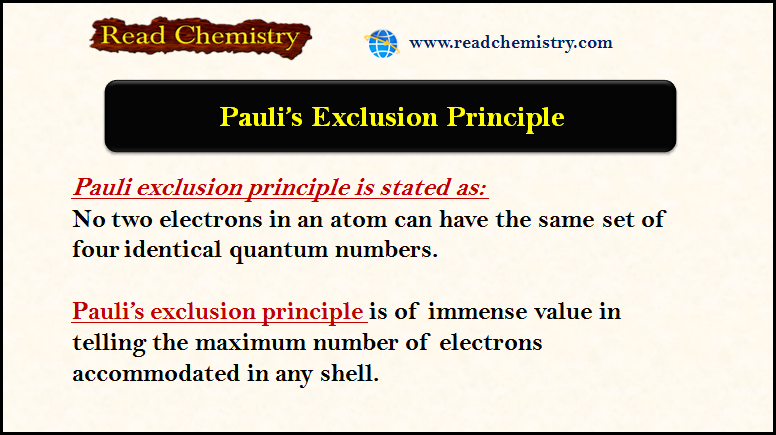
– Two electrons can occupy the same orbital only if they have opposite spins (Pauli’s exclusion principle).
Rule (5)
When several orbitals of equal energy (degenerate orbitals) are available, electrons prefer to occupy separate orbitals rather than getting paired in the same orbital.
– Such electrons tend to have same spins (Hund’srule).
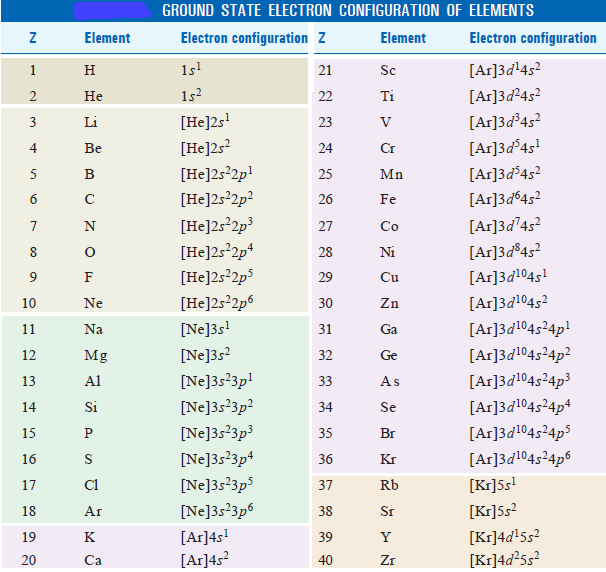
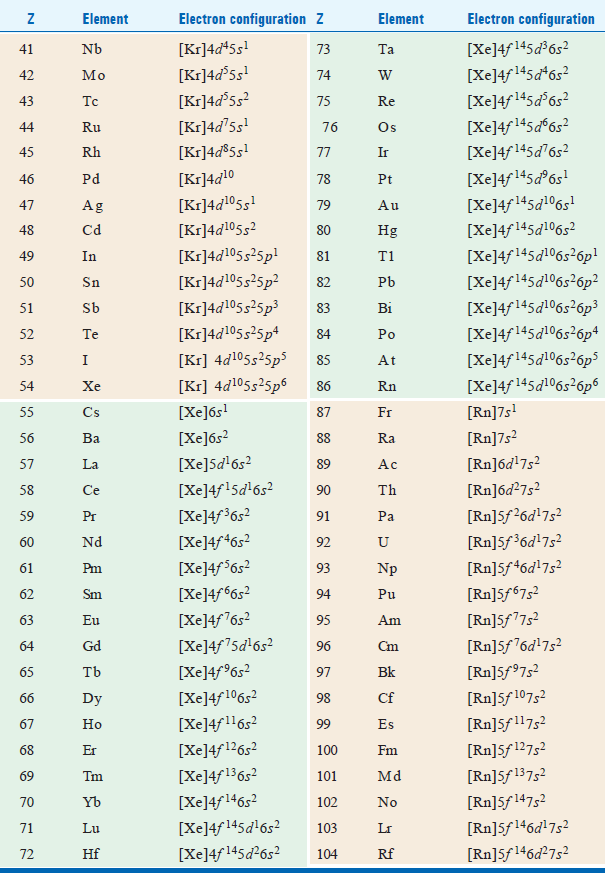
– Actual electron configuration of atoms of all elements of the periodic table is given in the following table:
– We find that these configurations are by and large the same as predicted by the Aufbau procedure.
Deviation from the standard pattern
– However, there are quite a few elements which exhibit slight variations from the standard pattern.
– Some anomalies are tabled below showing only the concerned orbitals.
– We find from the table that irregularities involve the placing of one or two electrons from ns orbital in (n – 1) d orbitals.
– There is very little energy difference between such (s) and (d) orbitals so that there is very little to choose from energy point of view.
– The deviations occur when (d) level orbitals are either almost full (e.g., Cu, Pd, Ag, Pt and Au) or half-full (Cr and Mo).
– The explanation for this deviation lies in the superior stability of completely filled or all half-filled orbitals than nearly filled or nearly half-filled orbitals.
– Thus d5 and d10 configurations are much more stable than d4 or d8 or d9.
– Spectroscopic data and magnetic properties of elements justify the statement that half-filled and completely filled subshells contribute to the stability.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.