The Effect of Electrolyte on Chemical Equilibria
The Effect of Electrolyte on Chemical Equilibria
– Experimentally, we find that the position of most solution equilibria depends on the electrolyte concentration of the medium, even when the added electrolyte contains no ion in common with those participating in the equilibrium.
– For example, consider again the oxidation of iodide ion by arsenic acid:
– If an electrolyte, such as barium nitrate, potassium sulfate, or sodium perchlorate, is added to this solution, the color of the triiodide ion becomes less intense.
– This decrease in color intensity indicates that the concentration of I3– has decreased and that the equilibrium has been shifted to the left by the added electrolyte.
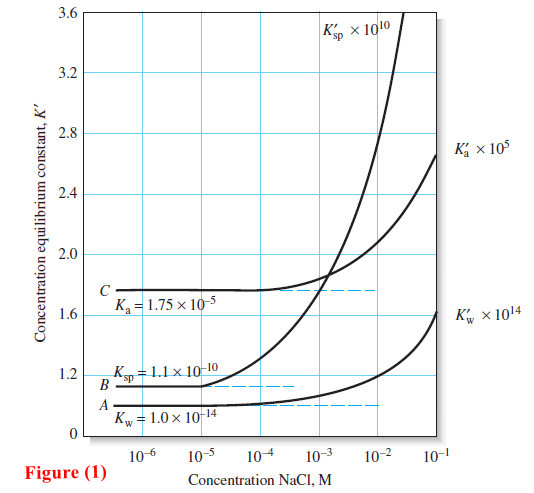
– Figure (1) further illustrates the effect of electrolytes.
– Curve A is a plot of the product of the molar hydronium and hydroxide ion concentrations (×1014) as a function of the concentration of sodium chloride. This concentration based ion product is designated K’w.
– At low sodium chloride concentrations, K’w becomes independent of the electrolyte concentration and is equal to 1.00 × 10-14, which is the thermodynamic ion-product constant for water, Kw (curve A, dashed line).
– A relationship that approaches a constant value as some variable (in this instance, the electrolyte concentration) approaches zero is called a limiting law.
– The constant numerical value observed at this limit is referred to as a limiting value.
– The vertical axis for curve B in Figure (1) is the product of the molar concentrations of barium and sulfate ions (×1010) in saturated solutions of barium sulfate.
This concentration-based solubility product is designated as K’sp.
– At low electrolyte concentrations, K’sp has a limiting value of 1.1 × 10-10, which is the accepted thermodynamic value of Ksp for barium sulfate.
– Curve C is a plot of Kra (×105), the concentration-based equilibrium constant for the acetic acid dissociation as a function of electrolyte concentration.
– We see once again that the ordinate function approaches a limiting value Ka = 1.75 × 10-5, which is the thermodynamic acid dissociation constant for acetic acid.
– The dashed lines in Figure (1) represent ideal behavior of the solutes.
– Note that departures from ideality can be significant. For example, the product of the molar concentrations of hydrogen and hydroxide ion increases from 1.0 × 10-14 in pure water to about 1.7 × 10-14 in a solution that is 0.1 M in sodium chloride, a 70% increase.
– The effect is even more pronounced with barium sulfate. In 0.1 M sodium chloride, the K’sp is more than double that of its limiting value.
– The electrolyte effect shown in Figure (1) is not unique to sodium chloride.
– In fact, we would see nearly identical curves if potassium nitrate or sodium perchlorate were substituted for sodium chloride.
– In each case, the origin of the effect is the electrostatic attraction between the ions of the electrolyte and the ions of reacting species of opposite charge.
– Since the electrostatic forces associated with all singly charged ions are approximately the same, the three salts exhibit essentially identical effects on equilibria.
– Next, we consider how we can take the electrolyte effect into account when we wish to make more accurate equilibrium calculations than those that you may have made in your previous work.
(1) The Effect of Ionic Charges on Equilibria
– Extensive studies have revealed that the magnitude of the electrolyte effect is highly dependent on the charges of the participants in an equilibrium.
– When only neutral species are involved, the position of equilibrium is essentially independent of electrolyte concentration.
– With ionic participants, the magnitude of the electrolyte effect increases with charge.
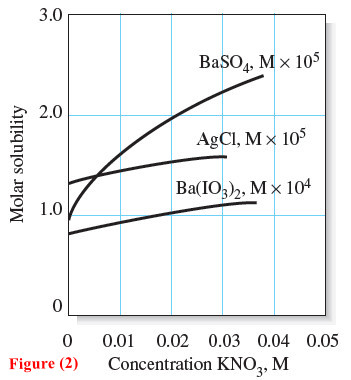
– This generality is demonstrated by the three solubility curves in Figure (2).
– Note, for example, that, in a 0.02 M solution of potassium nitrate, the solubility of barium sulfate with its pair of doubly charged ions is larger than it is in pure water by a factor of 2.
– This same change in electrolyte concentration increases the solubility of barium iodate by a factor of only 1.25 and that of silver chloride by 1.2.
– The enhanced effect due to doubly charged ions is also reflected in the greater slope of curve B in Figure (1).
(2) The Effect of Ionic Strength
– Systematic studies have shown that the effect of added electrolyte on equilibria is independent of the chemical nature of the electrolyte but depends on a property of the solution called the ionic strength. This quantity is defined as:
where [A], [B], [C], . . . represent the species molar concentrations of ions A, B, C, . . . and ZA, ZB, ZC, . . . are their charges.
Problem (1): Calculate the ionic strength of (a) a 0.1 M solution of KNO3 and (b) a 0.1 M solution of Na2SO4.
Solution:
Problem (2): What is the ionic strength of a solution that is 0.05 M in KNO3 and 0.1 M in Na2SO4?
Solution:
– These examples show that the ionic strength of a solution of a strong electrolyte consisting solely of singly charged ions is identical to its total molar salt concentration.
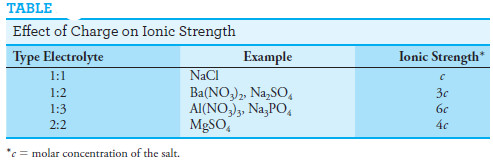
– The ionic strength is greater than the molar concentration, however, if the solution contains ions with multiple charges (see Table).
– For solutions with ionic strengths of 0.1 M or less, the electrolyte effect is independent of the kind of ions and dependent only on the ionic strength.
– Thus, the solubility of barium sulfate is the same in aqueous sodium iodide, potassium nitrate, or aluminum chloride provided the concentrations of these species are such that the ionic strengths are identical.
– Note that this independence with respect to electrolyte species disappears at high ionic strengths.
(3) The Salt Effect
– The electrolyte effect (also called the salt effect), which we have just described, results from the electrostatic attractive and repulsive forces between the ions of an electrolyte and the ions involved in an equilibrium.
– These forces cause each ion from the dissociated reactant to be surrounded by a sheath of solution that contains a slight excess of electrolyte ions of opposite charge.
– For example, when a barium sulfate precipitate is equilibrated with a sodium chloride solution, each dissolved barium ion tends to attract Cl– and repel Na+, therefore creating a slightly negative ionic atmosphere around the barium ion.
– Similarly, each sulfate ion is surrounded by an ionic atmosphere that tends to be slightly positive.
– These charged layers make the barium ions appear to be somewhat less positive and the sulfate ions somewhat less negative than in the absence of sodium chloride.
– The result of this shielding effect is a decrease in overall attraction between barium and sulfate ions and a corresponding increase in the solubility of BaSO4.
– The solubility becomes greater as the number of electrolyte ions in the solution becomes larger.
– In other words, the effective concentration of barium ions and of sulfate ions becomes less as the ionic strength of the medium becomes greater.
Reference: Fundamentals of analytical chemistry / Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. (ninth edition) , 2014 . USA