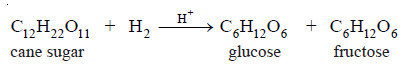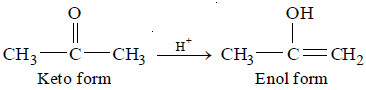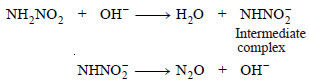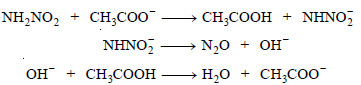Acid-Base catalysis (definition – Examples – Mechanism)
– In this topic, we will discuss Acid-Base catalysis: definition, Examples and Mechanism.
Acid-Base catalysis
– A number of homogeneous catalytic reactions are known which are catalysed by acids or bases, or both acids and bases. These are often referred to as Acid-Base catalysts.
– Arrhenius pointed out that acid catalysis was, in fact, brought about by H+ ions supplied by strong acids, while base catalysis was caused by OH– ions supplied by strong bases
Examples
(1) Inversion of Cane sugar
(2) Keto-Enol tautomerism of Acetone
(3) Decomposition of Nitramide
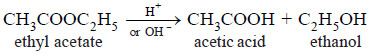
(4) Hydrolysis of an Ester
General Acid-Base catalysis
– More recently it has been found that :
(a) Not only H+ ions but all Bronsted bases (proton donors) cause acid catalysis.
– Thus the general acid catalysts are: H+, undissociated acids (CH3COOH), cations of weak bases (NH4+) , and water (H2O).
(b) Not only OH– ions but all Bronsted bases (proton acceptors) act as base catalyst.
– Thus the general base catalysts are: OH–, undissociated bases, anions of weak acids (CH3COO–) and water (H2O).
– The catalysis brought about by general acids and bases is termed General Acid-Base catalysis.
– For elucidation, decomposition of nitramide is also catalysed by acetate ions (CH3COO–).
Mechanism of Acid-Base catalysis
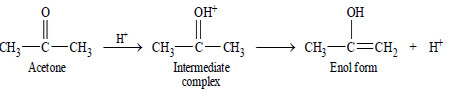
(a) In acid catalysis
– The H+ (or a proton donated by Bronsted acid) forms an intermediate complex with the reactant, which then reacts to give back the proton. For example, the mechanism of keto-enol tautomerism of acetone is :
(b) In base catalysis
– The OH– ion (or any Bronsted base) accepts a proton from the reactant to form an intermediate complex which then reacts or decomposes to regenerate the OH– (or Bronsted base).
– For example, the decomposition of nitramide by OH– ions and CH3COO– ions may be explained as follows :
(i) By OH– ions
(ii) By CH3COO– ions :