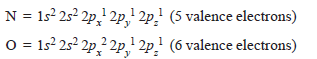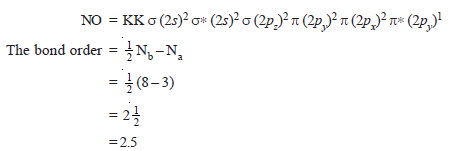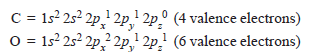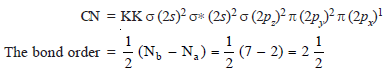Molecular Orbitals for Heteronuclear Diatomic Molecules
Molecular Orbitals for Heteronuclear Diatomic Molecules
– In the previous subject, we talk about but electronic structures and bonding properties of some of The homonuclear diatomic molecules. but in this subject, we will talk about Molecular Orbitals for Heteronuclear Diatomic Molecules (MO Theory)
– When two different atoms are bonded together, their molecule is called heteronuclear molecule.
– The same general principle applies to heteronuclear molecules but the main difference is that in heteronuclear molecules different atoms contribute unequally to the wave function i.e.
ψMO= C1 ψ1 AO + C2 ψ2 AO
– where C1 and C2 are two constants having different values for different atoms.
– Also the molecular orbitals formed are unsymmetrical due to difference in electronegativities.
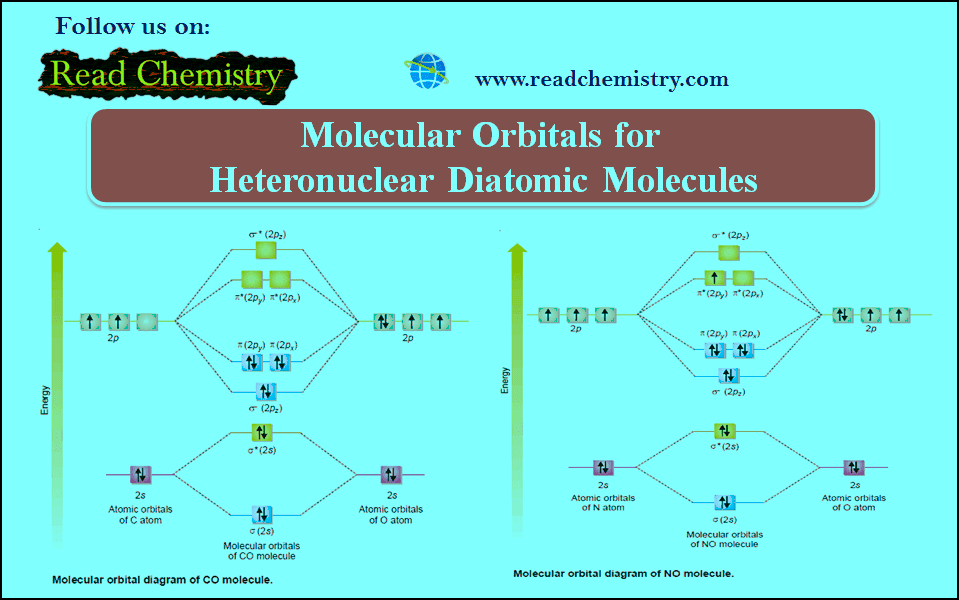
– In the construction of Molecular Orbital diagrams for heteronuclear molecules the bonding MOs are shown closed to electronegative atoms while antibonding MOs are shown closer to lesser electronegative atom.
– It may be noted if two different molecules have the same total number of electrons their molecular energy diagram will be similar.
– Let us now construct the molecular energy diagram for some heteronuclear molecules.
(1) Molecular Orbitals in Nitric Oxide, NO
– The electronic configuration of participating N and O atoms are:
– The total number of valence electrons is 11 and the electronic configuration of NO molecule can be written as:
– It makes clear that one σ bond and two pi bonds with an unpaired electron in antibonding π* (2py)1 molecular orbital are formed.
– This molecule is less stable than N2 molecule (Bond order = 3).
– Due to the presence of one unpaired electron in NO molecule it is paramagnetic is nature.
– The MO diagram for NO molecule is shown in the following figure:
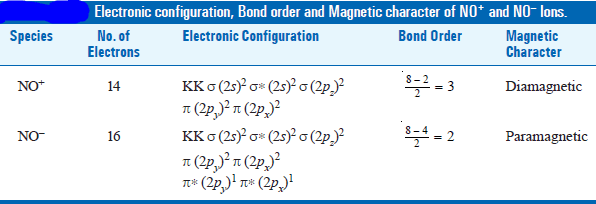
(2) Molecular Orbitals in NO+ and NO– Ions
– On similar lines, we can write the electronic configuration, bond order, and magnetic character of these ions.
– These are summarised in the following table:
(3) Carbon Monoxide, CO
– The electronic configurations of participating C and O atoms are:
– The total number of valence electrons is 10 and the electronic configuration of CO molecule can be written as:
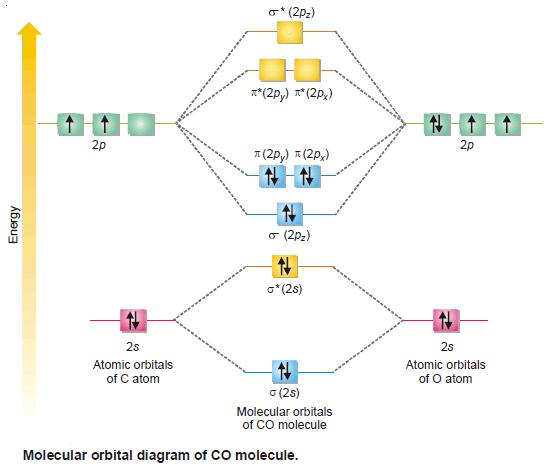
– This shows that carbon and oxygen atom in CO are bonded to each other by a triple bond (one σ bond and two π bonds).
– There is no unpaired electron in CO molecule and hence it is diamagnetic in nature.
– The molecular orbital diagram for CO molecule is shown in the following figure:
(4) CN Molecule
– The electronic configuration of participating C and N atoms are:

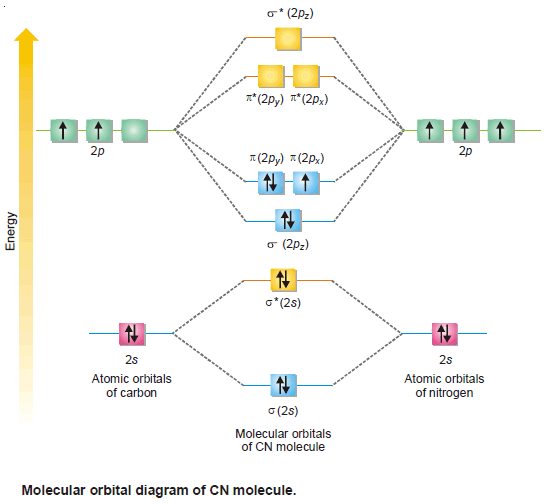
– The total number of valence electrons is 9 and the electronic configuration of CN molecule can be written as:
– Since the bond order in CN molecule is lesser than CO molecule, the former is less stable than the latter.
– Also, there is one unpaired electron in CN molecule. It is paramagnetic in nature.
– The molecular orbital diagram of CN molecule is shown in the following figure:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.