Covalent Bond: Definition, Examples, Types, Properties
– In this subject, we will discuss the Covalent Bond (Definition, Examples, Types, Properties)
Covalent Bond
– The electron transfer theory could not explain the bonding in molecules such as H2, O2, Cl2, etc., and in organic molecules, that had no ions.
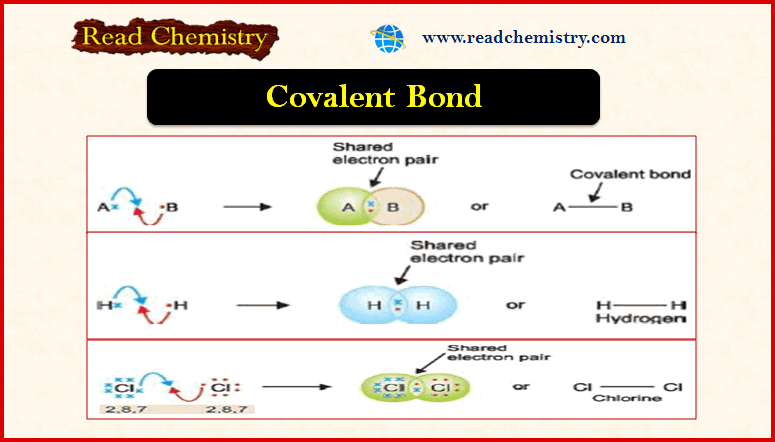
– It was G.N. Lewis who suggested that two atoms could achieve stable 2 or 8 electrons in the outer shell by sharing electrons between them.
– Let us consider a general case where atom A has one valence electron and another atom B has seven valence electrons.
– As they approach each other, each atom contributes one electron and the resulting electron pair fills the outer shell of both the atoms.
– Thus A acquires stable 2 electrons and B, 8 electrons in the outer shell.
– The shared pair is indicated by a dash (–) between the two bonded atoms.
– A shared pair of electrons constitutes a Covalent bond or Electron-pair bond.
– In fact, the positive nuclei of atoms A and B are pulled towards each other by the attraction of the shared electron pair.
– At the same time, the nuclei of two atoms also repel each other as do the two electrons.
– It is the net attractive force between the shared electrons and the nuclei that holds the atoms together.
– Thus an alternative definition of a covalent bond would be: The attractive force between atoms is created by the sharing of an electron pair.
– The compounds containing a covalent bond are called covalent compounds.
Conditions for the formation of Covalent Bond
– The conditions favorable for the formation of an ionic bond are:
(1) Number of valence electrons
Each of the atoms A and B should have 5, 6, or 7 valence electrons so that both achieve the stable octet by sharing a 3, 2, or 1 electron pair.
– H has one electron in the valence shell and attains a duplet.
– The non-metals of groups VA, VIA, and VIIA respectively satisfy this condition.
(2) Equal electronegativity
– The atom A will not transfer electrons to B if both have equal electronegativity and hence electron sharing will take place.
– This can be strictly possible only if both atoms are of the same element.
(3) Equal sharing of electrons
– The atoms A and B should have equal (or nearly equal) electron affinity so that they attract the bonding electron pair equally.
– Thus equal sharing of electrons will form a nonpolar covalent bond.
– Of course, precisely equal sharing of electrons will not ordinarily occur except when atoms A and B are atoms of the same element, for no two elements have exactly the same electron affinity.
Some examples of covalent compounds
– The construction of Lewis structures of simple covalent compounds will be discussed.
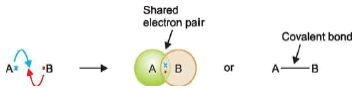
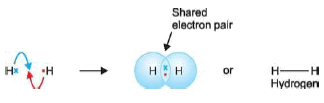
Covalent bond in Hydrogen, H2
– A hydrogen molecule is made of two H atoms, each having one valence electron.
– Each contributes an electron to the shared pair and both atoms acquire stable helium configuration.
– Thus stable H2 molecule results.
Covalent bond in Chlorine, Cl2
– Each Cl atom (2, 8, 7) has seven valence electrons.
– The two Cl atoms achieve a stable electron octet by sharing a pair of electrons.
Covalent bond in Water, H2O
– The oxygen atom (2, 6) has six valence electrons and can achieve a stable octet by sharing two electrons, one with each H atom.
– Thus Lewis’s structure of water can be written as:
Covalent bond in Ammonia, NH3
– Nitrogen atom (2, 5) has five valence electrons and can achieve the octet by sharing three electrons, one each with three H atoms.
– This gives the following Lewis structure for ammonia:
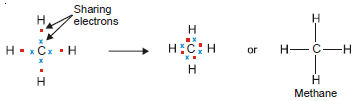
Covalent bond in Methane, CH4
– Carbon atoms (2, 4) have four electrons in the valence shell.
– It can achieve the stable octet by sharing these electrons with four H atoms, one with each H atom.
– Thus the Lewis structure of methane can be written as:
Examples of multiple covalent compounds
– In many molecules, we find that to satisfy the octet, it becomes necessary for two atoms to share two or three pairs of electrons between the same two atoms.
– The sharing of two pairs of electrons is known as a Double bond and the sharing of three pairs of electrons is a Triple bond.
– Let us consider some examples of compounds containing these multiple covalent bonds in their molecules.
Covalent bond in Oxygen, O2
– The conventional Lewis structure of oxygen is written by the sharing of two pairs of electrons between two O atoms (2, 6).
– In this way, both the O atoms achieve the octet
– The above structure of oxygen implies that all the electrons in oxygen, O2, are paired whereby the molecule should be diamagnetic.
– However, the experiment shows that O2 is paramagnetic with two unpaired electrons.
– This could be explained by the structure.
– Although writing Lewis structures work very well in explaining the bonding in most simple molecules, it should be kept in mind that it is simply the representation of a theory.
– In this case, the theory just doesn’t work.
Covalent bond in Nitrogen, N2
– The two atoms of nitrogen (2, 5), each having five electrons in the valence shell, achieve the octet by sharing three electron pairs between them.
Covalent bond in Carbon Dioxide, CO2
– Carbon (2, 4) has four valence electrons. It shares two electrons with each O atom (having six valence electrons).
– Thus the C atom and both the O atoms achieve their octet.
Properties of covalent compounds
– While the atoms in a covalent molecule are firmly held by the shared electron pair, the individual molecules are attracted to each other by weak van der Waals forces.
– Thus the molecules can be separated easily as not much energy is required to overcome the intermolecular attractions.
– This explains the general properties of covalent compounds.
(1) Gases, liquids, or solids at room temperature
– The covalent compounds are often gases, liquids, or relatively soft solids under ordinary conditions.
– This is so because of the weak intermolecular forces between the molecules.
(2) Low melting points and boiling points
– Covalent compounds have generally low melting points (or boiling points).
– The molecules are held together in the solid crystal lattice by weak forces.
– On application of heat, the molecules are readily pulled out and these then acquire kinetic energy for free movement as in a liquid.
– For the same reason, the liquid molecules are easily obtained in the gaseous form which explains the low boiling points of covalent liquids.
(3) Neither hard nor brittle
– While the ionic compounds are hard and brittle, covalent compounds are neither hard nor brittle.
– There are weak forces holding the molecules in the solid crystal lattice.
– A molecular layer in the crystal easily slips relative to other adjacent layers and there are no ‘forces of repulsion’ like those in ionic compounds.
– Thus the crystals are easily broken and there is no sharp cleavage between the layers on application of external force.
(4) Soluble in organic solvents
– In general, covalent compounds dissolve readily in nonpolar organic solvents (benzene, ether).
– The kinetic energy of the solvent molecules easily overcomes the weak intermolecular forces.
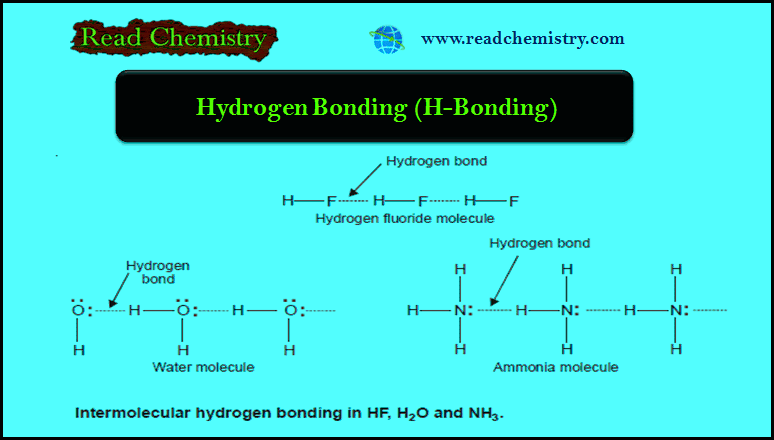
– Covalent compounds are insoluble in water. Some of them (alcohols, and amines) dissolve in water due to hydrogen bonding.
(5) Non-conductors of electricity
– Since there are no (+) or (–) ions in covalent molecules, the covalent compounds in the molten or solution form are incapable of conducting electricity.
(6) Exhibit Isomerism
– Covalent bonds are rigid and directional, the atoms being held together by a shared electron pair and not by electrical lines of force.
– This affords the opportunity for various spatial arrangements and covalent compounds to exhibit stereoisomerism.
(7) Molecular reactions
– The covalent compounds give reactions where the molecule as a whole undergoes a change.
– Since there are no strong electrical forces to speed up the reaction between molecules, these reactions are slow.
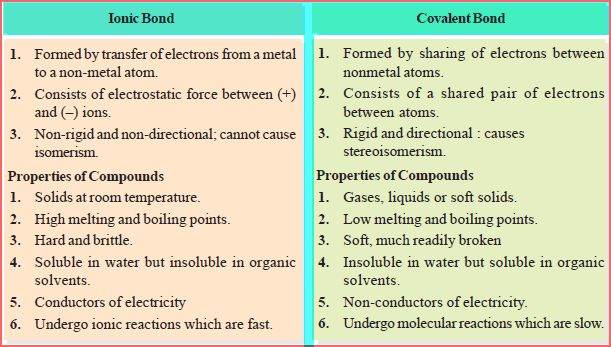
Difference between a covalent and ionic bond
– The following table shows the Comparison of Ionic bond and covalent bond
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.