Measurement of The Heat of Reaction
Measurement of The Heat of Reaction
– The experimental measurement of the heat of reaction or enthalpy change is known as calorimetry.
– The name (calorimetry) evidently finds its origin in the unit of heat–the calorie.
– The heat given out or absorbed in a chemical reaction is measured in a suitable apparatus called a calorimeter.
– These calorimeters vary considerably in their construction and designs.
– They are adapted to suit the requirements of a particular reaction under study.
– For instance, to measure the heats of reactions involving (i) solutions only, (ii) gases, (iii) very reactive chemicals etc., different types of calorimeters are employed.
– We discuss below two of the common types of calorimeters.
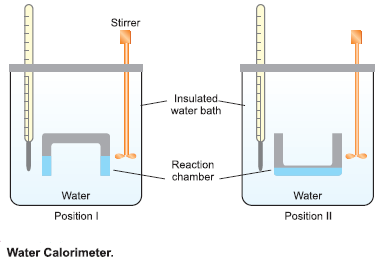
Water Calorimeter
– This is a convenient apparatus for finding the heat changes accompanying chemical reactions taking place in solutions.
– The apparatus consists essentially of a water bath with thermally insulated walls.
– A reaction chamber consisting of two limbs is suspended in the water bath.
– Through the lid of the water bath pass:
(a) thermometer that records the temperature variations.
(b) a stirrer that stirs water in the water bath.
– A known quantity of water (say W gms) is taken in the water bath and its temperature is noted.
– The reacting substances are filled in the two limbs as shown in the Figure above.
– The reacting chamber is now turned upside down (position II) to allow the solutions to mix.
– They react and the heat produced during the reaction is taken up by water, raising its temperature.
– If the rise in temperature (Final reading – Initial reading) is t ºC, the heat absorbed by water ‘Q’ is given by:
Q = W × 1 × t calories
– But heat produced in the reaction is equal to that absorbed by water, hence heat of the reaction can be calculated.
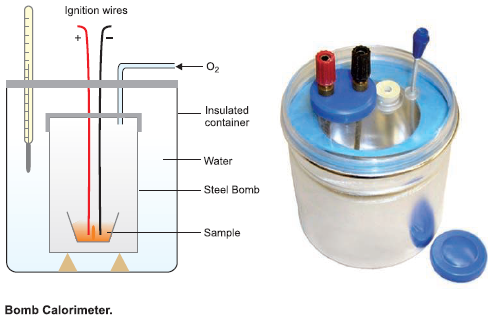
Bomb Calorimeter
– This apparatus was devised by Berthelot (1881) to measure the heat of combustion of organic compounds.
– A modified form of the apparatus is shown in the following figure:
– The apparatus consists of a sealed combustion chamber, called a bomb, containing a weighed quantity of the substance in a dish along with oxygen under about 20 atm pressure.
– The bomb is lowered in water contained in an insulated copper vessel.
– This vessel is provided with a stirrer and a thermometer reading up to 1/100th of a degree.
– It is also surrounded by an outer jacket to ensure complete insulation from the atmosphere.
– The temperature of water is noted before the substance is ignited by an electric current.
– After combustion, the rise in temperature of the system is noted on the thermometer, and the heat of combustion can be calculated from the heat gained by water and the calorimeter.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.