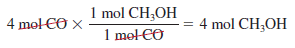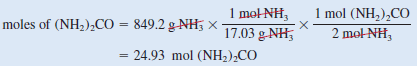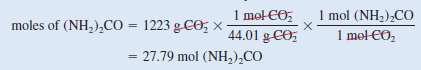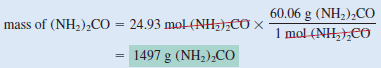Limiting Reagent: Definition, Examples, Problems
– In this subject, we will discuss the Limiting Reagent (Definition, Examples, Problems)
Limiting Reagent
– When a chemist carries out a reaction, the reactants are usually not present in exact stoichiometric amounts, that is, in the proportions indicated by the balanced equation.
– Because the goal of a reaction is to produce the maximum quantity of a useful compound from the starting materials, frequently a large excess of one reactant is supplied to ensure that the more expensive reactant is completely converted to the desired product.
– Consequently, some reactants will be left over at the end of the reaction.
– The reactant used up first in a reaction is called the limiting reagent because the maximum amount of product formed depends on how much of this reactant was originally present.
– When this reactant is used up, no more product can be formed.
– Excess reagents are the reactants present in quantities greater than necessary to react with the quantity of the limiting reagent.
The concept of limiting reagent
– The concept of the limiting reagent is analogous to the relationship between men and women in a dance contest at a club.
– If there are 14 men and only 9 women, then only 9 female/male pairs can compete. Five men will be left without partners.
– The number of women thus limits the number of men that can dance in the contest, and there is an excess of men.
Example: synthesis of methanol (CH3OH)
– Consider the industrial synthesis of methanol (CH3OH) from carbon monoxide and hydrogen at high temperatures:
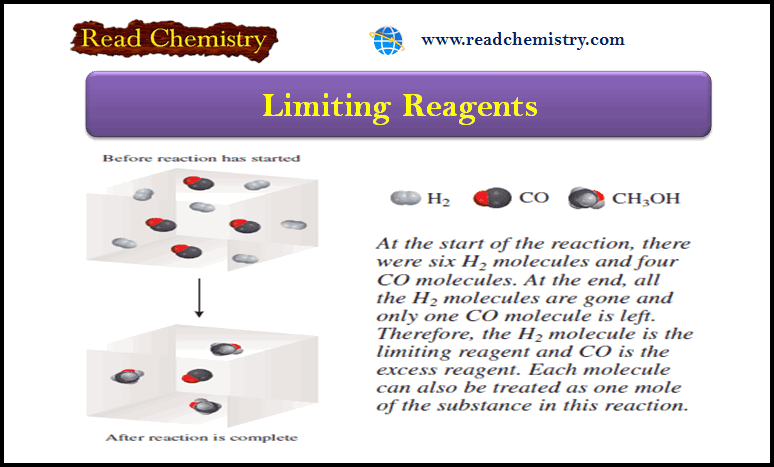
– Suppose initially we have 4 moles of CO and 6 moles of H2 (see Figure above).
– One way to determine which of the two reactants is the limiting reagent is to calculate the number of moles of CH3OH obtained based on the initial quantities of CO and H2.
– From the preceding definition, we see that only the limiting reagent will yield a smaller amount of the product.
– Starting with 4 moles of CO, we find the number of moles of CH3OH produced is:
– and starting with 6 moles of H2, the number of moles of CH3OH formed is:
– Because H2 results in a smaller amount of CH3OH, it must be the limiting reagent.
– Therefore, CO is the excess reagent.
Note
– In stoichiometric calculations involving limiting reagents, the first step is to decide which reactant is the limiting reagent.
– After the limiting reagent has been identified, the rest of the problem can be solved.
– the following Example illustrates this approach.
Solved Problems on Limiting Reagent
Urea [(NH2)2CO] is prepared by reacting ammonia with carbon dioxide:
In one process, 849.2 g of NH3 are treated with 1223 g of CO2.
(a) Which of the two reactants is the limiting reagent?
(b) Calculate the mass of (NH2)2CO formed.
(c) How much excess reagent (in grams) is left at the end of the reaction?
(A) Strategy
– The reactant that forms fewer moles of product is the limiting reagent because it limits the amount of product that can be formed.
– How do we convert from the amount of reactant to the amount of product?
– Perform this calculation for each reactant, then compare the moles of product, (NH2)2CO, formed by the given amounts of NH3 and CO2 to determine which reactant is the limiting reagent.
Solution:
– We carry out two separate calculations.
– First, starting with 849.2 g of NH3, we calculate the number of moles of (NH2)2CO that could be produced if all the NH3reacted according to the following conversions:
– Combining these steps into one step, we write:
– Second, for 1223 g of CO2, the conversions are:
– The number of moles of (NH2)2CO that could be produced if all the CO2reacted is:
– It follows, therefore, that NH3 must be the limiting reagent because it produces a smaller amount of (NH2)2CO.
(B) Strategy
– We determined the moles of (NH2)2CO produced in part (a), using NH3 as the limiting reagent.
– How do we convert from moles to grams?
Solution:
– The molar mass of (NH2)2CO is 60.06 g.
– We use this as a conversion factor to convert from moles of (NH2)2CO to grams of (NH2)2CO:
Check:
Does your answer seem reasonable? 24.93 moles of the product are formed. What is the mass of 1 mole of (NH2)2CO?
(C) Strategy
– Working backward, we can determine the amount of CO2 that reacted to produce 24.93 moles of (NH2)2CO.
– The amount of CO2 left over is the difference between the initial amount and the amount reacted.
Solution:
– Starting with 24.93 moles of (NH2)2CO, we can determine the mass of CO2 that reacted using the mole ratio from the balanced equation and the molar mass of CO2.
– The conversion steps are:
The amount of CO2 remaining (in excess) is the difference between the initial amount (1223 g) and the amount reacted (1097 g):
Check:
– Is this answer reasonable? Can the mass of CO2 remaining to be greater than the initial mass of CO2 used in the reaction?
– The last example brings out an important point.
– In practice, chemists usually choose the more expensive chemical as the limiting reagent so that all or most of it will be consumed in the reaction.
– In the synthesis of urea, NH2 is invariably the limiting reagent because it is much more expensive than CO2
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.