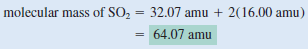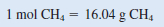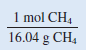General Chemistry
The Molecular Mass and The Mass Spectrometer
Molecular Mass
** If we know the atomic masses of the component atoms, we can calculate the mass of a molecule.
** The molecular mass: (sometimes called molecular weight) is the sum of the atomic masses (in amu) in the molecule.
** For example, the molecular mass of H2O is:
** In general, we need to multiply the atomic mass of each element by the number of atoms of that element present in the molecule and sum over all the elements.
Problem (1): Calculate the molecular masses (in amu) of the following compounds:
(a) sulfur dioxide (SO2)
(b) caffeine (C8H10N4O2)
Strategy:
How do atomic masses of different elements combine to give the molecular mass of a compound?
Solution:
To calculate molecular mass, we need to sum all the atomic masses in the molecule. For each element, we multiply the atomic mass of the element by the number of atoms of that element in the molecule. We find atomic masses in the periodic table (inside front cover).
(a) There are two O atoms and one S atom in SO2, so that:
(b) There are eight C atoms, ten H atoms, four N atoms, and two O atoms in caffeine, so the molecular mass of C8H10N4O2 is given by:
Notes
** From the molecular mass we can determine the molar mass of a molecule or compound.
** The molar mass of a compound (in grams) is numerically equal to its molecular mass (in amu). For example, the molecular mass of water is 18.02 amu, so its molar mass is 18.02 g.
** Note that 1 mole of water weighs 18.02 g and contains 6.022 × 1023 H2O molecules, just as 1 mole of elemental carbon contains 6.022 × 1023 carbon atoms.
** As the following Examples show, a knowledge of the molar mass enables us to calculate the numbers of moles and individual atoms in a given quantity of a compound.
Problem (2): Methane (CH4) is the principal component of natural gas. How many moles of CH4 are present in 4.83 g of CH4?
Strategy:
We are given grams of CH4 and asked to solve for moles of CH4. What conversion factor do we need to convert between grams and moles? Arrange the appropriate conversion factor so that grams cancel and the unit moles are obtained for your answer.
Solution:
The conversion factor needed to convert between grams and moles is the molar mass. First we need to calculate the molar mass of CH4
Because
the conversion factor we need should have grams in the denominator so that the unit g will cancel, leaving the unit mol in the numerator:
We now write:
Thus, there is 0.301 mole of CH4 in 4.83 g of CH4.
Check:
Should 4.83 g of CH4 equal less than 1 mole of CH4? What is the mass of 1 mole of CH4?
Problem (3): How many hydrogen atoms are present in 43.8 g of urea [(NH2)2CO], which is used as a fertilizer, in animal feed, and in the manufacture of polymers? The molar mass of urea is 60.06 g?
Strategy:
We are asked to solve for atoms of hydrogen in 43.8 g of urea. We cannot convert directly from grams of urea to atoms of hydrogen. How should molar mass and Avogadro’s number be used in this calculation? How many moles of H are in 1 mole of urea?
Solution:
** To calculate the number of H atoms, we first must convert grams of urea to moles of urea using the molar mass of urea.
** The molecular formula of urea shows there are four moles of H atoms in one mole of urea molecule, so the mole ratio is 4:1.
** Finally, knowing the number of moles of H atoms, we can calculate the number of H atoms using Avogadro’s number.
** We need two conversion factors: molar mass and Avogadro’s number. We can combine these conversions:
into one step:
Check:
Does the answer look reasonable? How many atoms of H would 60.06 g of urea contain?
Notes
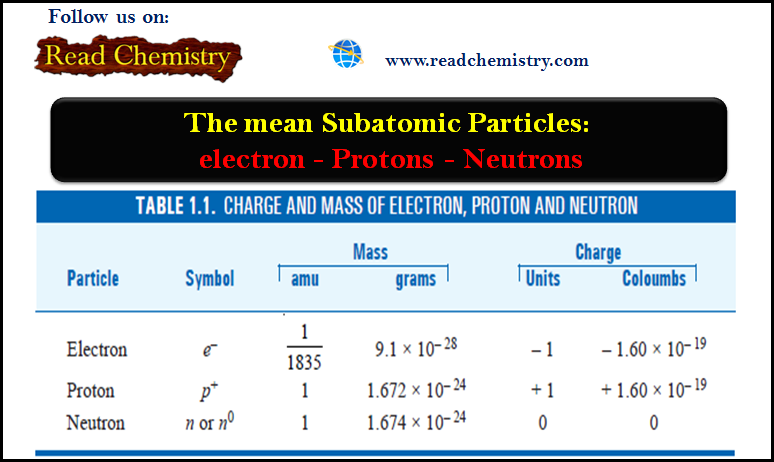
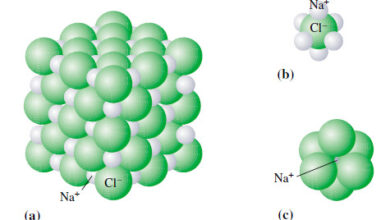
** For ionic compounds like NaCl and MgO that do not contain discrete molecular units, we use the term formula mass instead.
** The formula unit of NaCl consists of one Na+ ion and one Cl– ion. Thus, the formula mass of NaCl is the mass of one formula unit:
and its molar mass is 58.44 g.
The Mass Spectrometer
** The most direct and most accurate method for determining atomic and molecular
masses is mass spectrometry.
** The figure shows Schematic diagram of one type of mass spectrometer
** In a mass spectrometer:
(1) a gaseous sample is bombarded by a stream of high-energy electrons.
(2) Collisions between the electrons and the gaseous atoms (or molecules) produce positive ions by dislodging an electron from each atom or molecule.
(3) These positive ions (of mass m and charge e) are accelerated by two oppositely charged plates as they pass through the plates.
(4) The emerging ions are deflected into a circular path by a magnet. The radius of the path depends on the charge-to-mass ratio (that is, eym).
(5) Ions of smaller eym ratio trace a wider curve than those having a larger eym ratio, so that ions with equal charges but different masses are separated from one another.
(6) The mass of each ion (and hence its parent atom or molecule) is determined from the magnitude of its deflection. Eventually the ions arrive at the detector, which registers a current for eachtype of ion.
(7) The amount of current generated is directly proportional to the number of ions, so it enables us to determine the relative abundance of isotopes.
** The first mass spectrometer, developed in the 1920s by the English physicist F. W. Aston, was crude by today’s standards. Nevertheless, it provided indisputable evidence of the existence of isotopes—neon-20 (atomic mass 19.9924 amu and natural abundance 90.92 percent) and neon-22 (atomic mass 21.9914 amu and natural abundance 8.82 percent).
** When more sophisticated and sensitive mass spectrometers became available, scientists were surprised to discover that neon has a third stable isotope with an atomic mass of 20.9940 amu and natural abundance 0.257 percent (Figure shows The mass spectrum of the three isotopes of neon ).
** This example illustrates how very important experimental accuracy is to a quantitative science like chemistry. Early experiments failed to detect neon-21 because its natural abundance is just 0.257 percent. In other words, only 26 in 10,000 Ne atoms are neon-21. The masses of molecules can be determined in a similar manner by the mass spectrometer.
Reference: General Chemistry: The Essential Concepts / Raymond Chang , Jason Overby. (sixth edition).