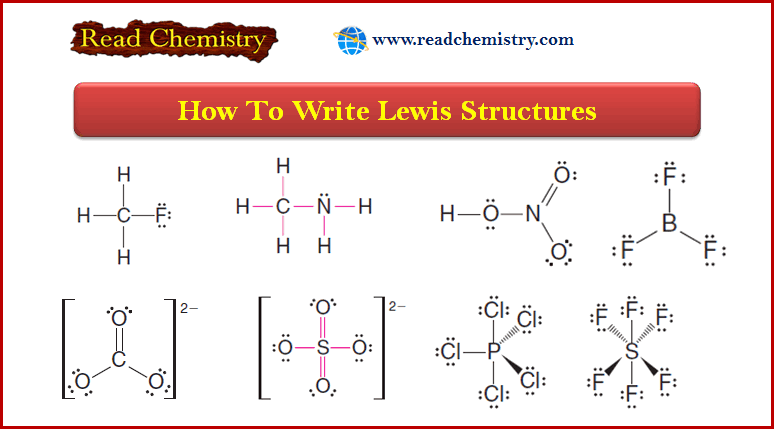
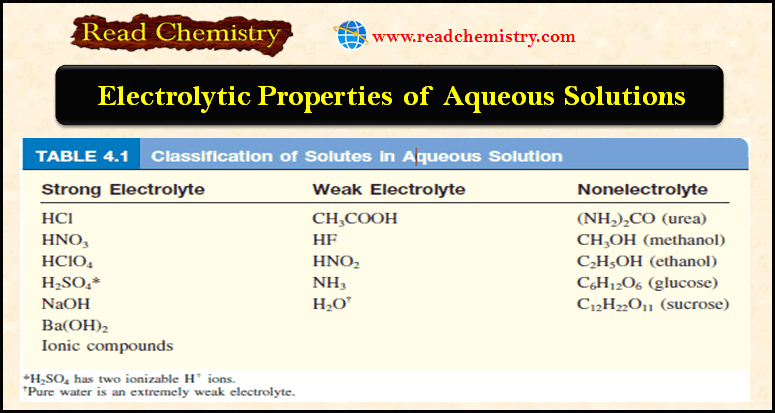
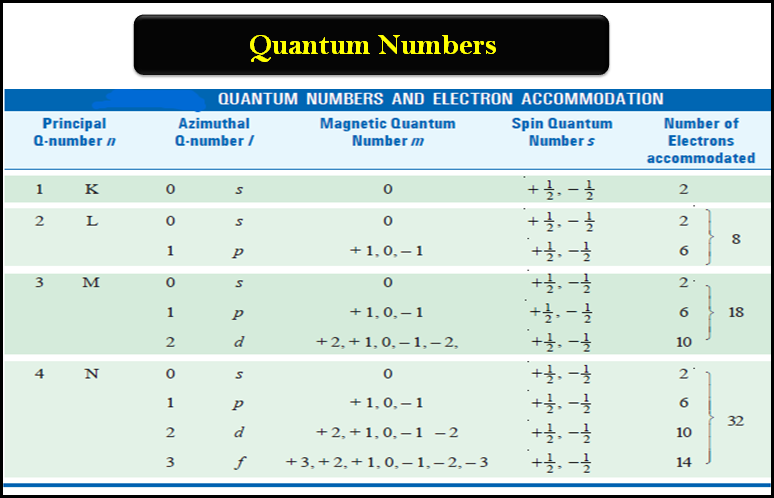
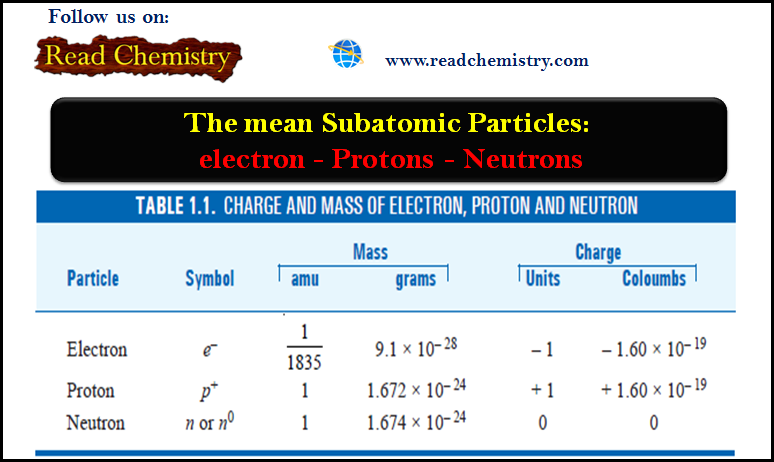
Molecular Formulas and Empirical Formulas: Definitions, Examples
– In this subject, we will discuss Molecular Formulas and Empirical Formulas: Definitions, Examples
– Chemists use Chemical formulas to express the composition of molecules and ionic compounds in terms of chemical symbols.
– By composition we mean not only the elements present but also the ratios in which the atoms are combined.
– Here we are mainly concerned with two types of formulas: molecular formulas and empirical formulas.
Molecular Formulas
– A molecular formulas shows the exact number of atoms of each element in the smallest unit of a substance.
– In our discussion of molecules, each example was given with its molecular formula in parentheses.
– Thus, H2 is the molecular formula for hydrogen, O2 is oxygen, O3 is ozone, and H2O is water.
– The subscript numeral indicates the number of atoms of an element present.
– There is no subscript for O in H2O because there is only one atom of oxygen in a molecule of water, and so the number “one” is omitted from the formula.
– Note that oxygen (O2) and ozone (O3) are allotropes of oxygen.
– An allotrope is one of two or more distinct forms of an element.
– Two allotropic forms of the element carbon—diamond and graphite—are dramatically different not only in properties but also in their relative cost.
Molecular Models
– Molecules are too small for us to observe directly.
– An effective means of visualizing them is the use of molecular models.
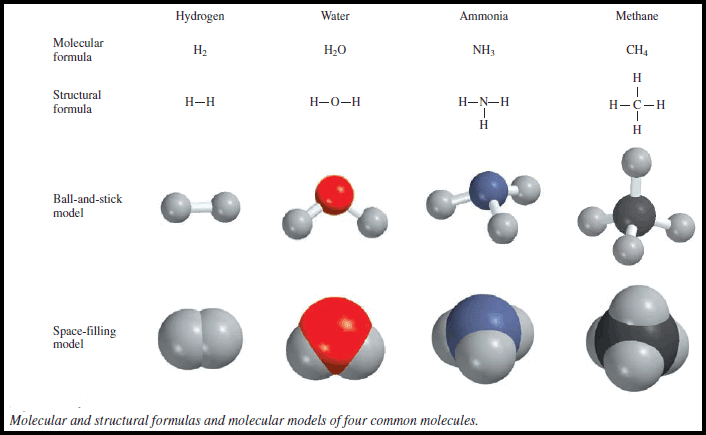
– Two standard types of molecular models are currently in use: ball-and-stick models and space-filling models (Figure).
(1) ball and- stick models
– In ball and- stick model kits, the atoms are wooden or plastic balls with holes in them.
– Sticks or springs are used to represent chemical bonds.
– The angles they form between atoms approximate the bond angles in actual molecules.
– Except for the H atom, the balls are all the same size and each type of atom is represented by a specific color.
(2) space-filling models
– In space-filling models, atoms are represented by truncated balls held together by snap fasteners, so that the bonds are not visible.
– The balls are proportional in size to atoms.
– The first step toward building a molecular model is writing the structural formula, which shows how atoms are bonded to one another in a molecule.
– For example, it is known that each of the two H atoms is bonded to an O atom in the water molecule.
– Therefore, the structural formula of water is H-O-H.
– A line connecting the two atomic symbols represents a chemical bond.
Difference between ball and- stick models and space-filling models
– Ball-and-stick models show the three-dimensional arrangement of atoms clearly, and they are fairly easy to construct.
– However, the balls are not proportional to the size of atoms.
– Furthermore, the sticks greatly exaggerate the space between atoms in a molecule.
– Space-filling models are more accurate because they show the variation in atomic size.
– Their drawbacks are that they are time-consuming to put together and they do not show the three-dimensional positions of atoms very well.
Empirical Formulas
– The empirical formula tells us which elements are present and the simplest whole-number ratio of their atoms, but not necessarily the actual number of atoms in a given molecule.
– The empirical formulas are the simplest chemical formulas;
– They are written by reducing the subscripts in the molecular formulas to the smallest possible whole numbers.
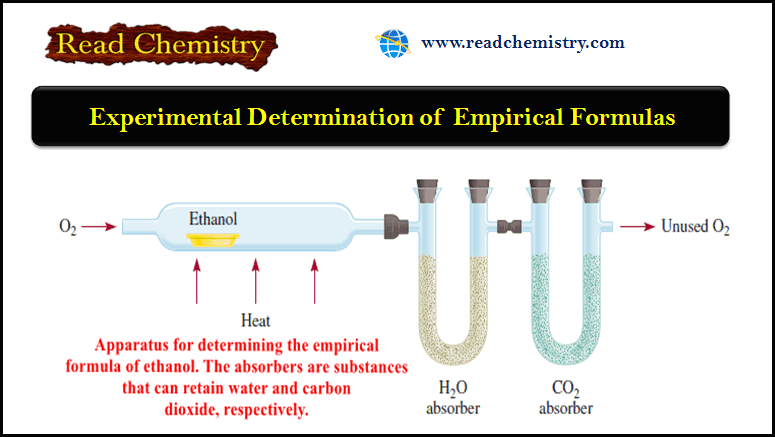
– The word “empirical” means “derived from experiment.”
– As we will see later, empirical formulas are determined experimentally.
Example(1): H2O2
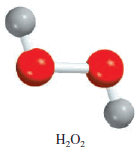
– The molecular formula of hydrogen peroxide, a substance used as an antiseptic and as a bleaching agent for textiles and hair, is H2O2.
– This formula indicates that each hydrogen peroxide molecule consists of two hydrogen atoms and two oxygen atoms.
– The ratio of hydrogen to oxygen atoms in this molecule is 2:2 or 1:1.
– The empirical formula of hydrogen peroxide is HO.
Example(2): N2H4
– The compound hydrazine (N2H4), which is used as a rocket fuel.
– The empirical formula of hydrazine is NH2.
– Although the ratio of nitrogen to hydrogen is 1:2 in both the molecular formula (N2H4) and the empirical formula (NH2), only the molecular formula tells us the actual number of N atoms (two) and H atoms (four) present in a hydrazine molecule.
Molecular Formulas and Empirical Formulas
– Molecular formulas are the true formulas of molecules.
– If we know the molecular formula, we also know the empirical formula, but the reverse is not true.
– Why, then, do chemists bother with empirical formulas? , when chemists analyze an unknown compound, the first step is usually the determination of the compound’s empirical formula.
– With additional information, it is possible to deduce the molecular formula.
– For many molecules, the molecular formula and empirical formula are the same.
– Some examples are water (H2O), ammonia (NH3), carbon dioxide (CO2), and methane (CH4).
Formula of Ionic Compounds
– The formulas of ionic compounds are usually the same as their empirical formulas because ionic compounds do not consist of discrete molecular units.
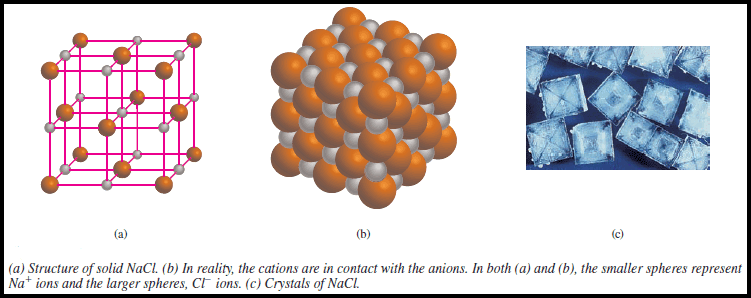
– For example, a solid sample of sodium chloride (NaCl) consists of equal numbers of Na+ and Cl– ions arranged in a three-dimensional network (Figure).
– In such a compound, there is a 1:1 ratio of cations to anions so that the compound is electrically neutral.
– As you can see in the Figure above, no Na+ ion in NaCl is associated with just one particular Cl– ion.
– Each Na+ ion is equally held by six surrounding Cl– ions and vice versa.
– Thus, NaCl is the empirical formula for sodium chloride.
– In other ionic compounds, the actual structure may be different, but the arrangement of cations and anions is such that the compounds are all electrically neutral.
– Note that the charges on the cation and anion are not shown in the formula for an ionic compound.
– For ionic compounds to be electrically neutral, the sum of the charges on the cation and anion in each formula unit must be zero.
– If the charges on the cation and anion are numerically different, we apply the following rule to make the formula electrically neutral: The subscript of the cation is numerically equal to the charge on the anion, and the subscript of the anion is numerically equal to the charge on the cation.
– If the charges are numerically equal, then no subscripts are necessary.
– This rule follows from the fact that because the formulas of most ionic compounds are empirical, the subscripts must always be reduced to the smallest ratios.
– Let us consider some examples:
(1) Potassium Bromide
– The potassium cation K+ and the bromine anion Br– combine to form the ionic compound potassium bromide.
– The sum of the charges is +1 + (-1) = 0, so no subscripts are necessary.
– The formula is KBr.
(2) Zinc Iodide
– The zinc cation Zn2+ and the iodine anion I– combine to form zinc iodide.
– The sum of the charges of one Zn2+ ion and one I– ion is +2 + (-1) = +1.
– To make the charges add up to zero we multiply the -1 charge of the anion by 2 and add the subscript “2” to the symbol for iodine.
– Therefore, the formula for zinc iodide is ZnI2.
(3) Aluminum Oxide
– The cation is Al3+ and the oxygen anion is O2-.
– The following diagram helps us determine the subscripts for the
– The compound is formed by the cation and the anion.
– The sum of the charges is 2(+3) + 3(-2) = 0.
– Thus, the formula for aluminum oxide is Al2O3.
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. ( sixth edition) .