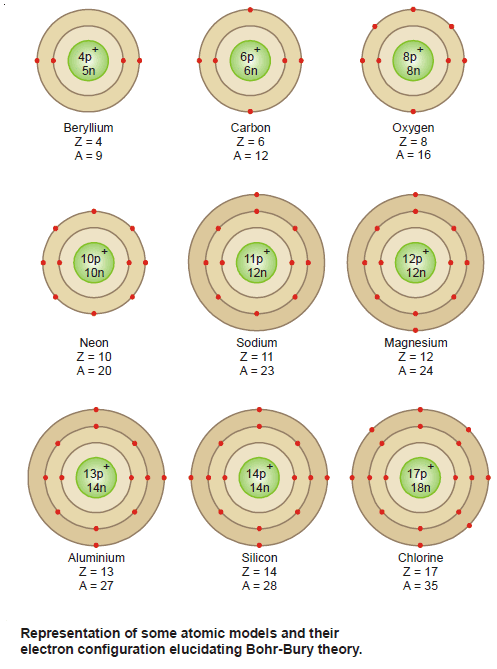
Bohr-Bury Scheme – Electron arrangement in the orbits
– The Bohr-Bury scheme considers that the maximum number of electrons that each orbit can contain is 2 × n2, where n is the number of orbits.
– Having known that planetary electrons numerically equal to the atomic number are revolving about the atomic nucleus in closed orbits, the question arises as to how they are arranged in these orbits.
Langmuir Scheme
– We are indebted to Langmuir for putting forward the first elaborate scheme of the arrangement of extranuclear electrons in 1919.
– His fundamental conception is that inert gases possess the most stable electron configuration and, therefore, contain complete electron orbits.
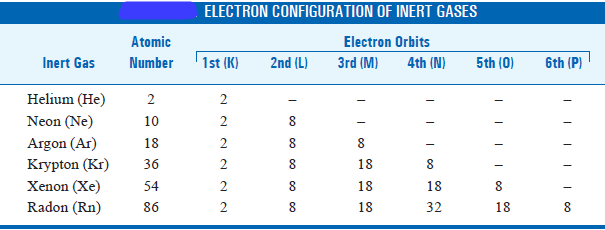
– Since helium has two planetary electrons, the first orbit is considered fully saturated with 2 electrons.
– In the next inert gas neon, we have 10 planetary electrons and since 2 electrons would fully saturate the first orbit the remaining 8 will form the next stable orbit.
– Argon with atomic number 18 will similarly have the similar arrangement 2, 8, 8.
– Proceeding in this manner the successive orbits would contain 2, 8, 8, 18, 32 electrons.
– Langmuir’s scheme although quite correct for the first few elements, failed to explain the behavior of higher elements.
Bohr-Bury Scheme
– In 1921, Bury put forward a modification of the Langmuir scheme which is in better agreement with the physical and chemical properties of certain elements.
– At about the same time as Bury developed his scheme on chemical grounds, Bohr (1921) published independently an almost identical scheme of the arrangement of extra-nuclear electrons.
– He based his conclusions on a study of the emission spectra of the elements.
– Bohr-Bury scheme as it may be called, can be summarised as follows:
Rule (1)
– The maximum number of electrons that each orbit can contain is 2 × n2, where n is the number of orbits.
– The first orbit can contain 2 × 12 = 2 ; second 2 × 22= 8 ; third 2 × 32 = 18 ; fourth 2 × 42 = 32, and so on.
Rule (2)
– The maximum number of electrons in the outermost orbit is 8 and in the next-to-the outermost 18.
Rule (3)
– It is not necessary for an orbit to be completed before another commences to be formed.
– In fact, a new orbit begins when the outermost orbit attains 8 electrons.
Rule (4)
– The outermost orbit cannot have more than 2 electrons and next-to-outermost cannot have more than eight so long as the next inner orbit, in each case, has not received the maximum electrons as required by rule (1).
– According to the Bohr-Bury scheme the configuration of the inert gases is given in the table below:
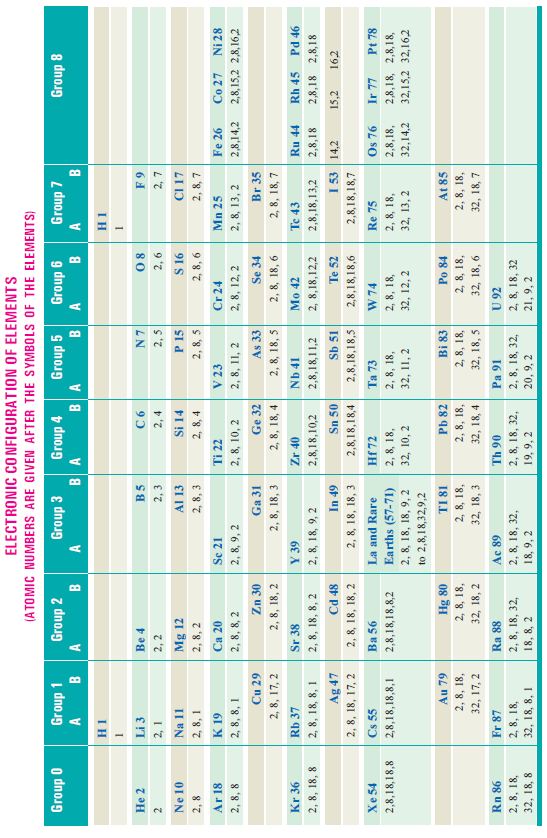
– A complete statement of the electron configuration of elements elucidating the various postulates of the Bohr-Bury scheme is given in the following table for ready reference.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.