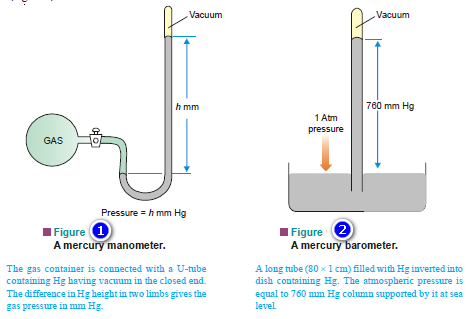
Fundamentals of Electrochemistry book by V.S. Bagotsky
– In this subject, we will discuss free download of Fundamentals of Electrochemistry book by V.S. Bagotsky
The Preface of Fundamentals of Electrochemistry book
– Two very important fields of natural science—chemistry and the science of electricity— matured and grew vigorously during the first half of the nineteenth century. Electrochemistry developed simultaneously.
– From the very beginning, electrochemistry was not merely a peripheral field but evolved with an important degree of independence, and it also left very significant marks on the development of chemistry and of the theory of electricity.
– Today, electrochemistry is a rigorous science concerned with the quantitative relations among the chemical, surface, and electrical properties of systems.
– Electrochemistry has strong links to many other fields of science.
– Electrochemical concepts proved particularly fruitful for studying and interpreting a number of very important biological processes
– This book seeks essentially to provide a rigorous, yet lucid and comprehensible outline of the basic concepts (phenomena, processes, and laws) that form the subject matter of modern theoretical and applied electrochemistry.
– Particular attention is given to electrochemical problems of fundamental significance, yet those often treated in an obscure or even incorrect way in monographs and texts.
– Among these problems are some, that appear elementary at first glance, such as the mechanism of current flow in electrolyte solutions, the nature of electrode potentials, and the values of the transport numbers in diffusion layers.
Description of Fundamentals of Electrochemistry book
Book name:
Fundamentals of Electrochemistry by Vladimir Sergeevich Bagotsky.
Author:
- Vladimir Sergeevich Bagotsky (V. S. BAGOTSKY)
A. N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
Edition: second edition
Year: 2006
No of Chapters: 37
No of Pages: 719
File format: pdf
File size: 5.98 MB
Contents of Fundamentals of Electrochemistry book
PART (I): BASIC CONCEPTS
Chapter 1: Electric Currents in Ionic Conductors
Chapter 2: Electrode Potentials
Chapter 3: Thermodynamics of Electrochemical Systems
Chapter 4: Mass Transfer in Electrolytes
Chapter 5: Phase Boundaries (Interfaces) Between Miscible Electrolytes
Chapter 6: Polarization of Electrodes
Chapter 7: Aqueous Electrolyte Solutions
Chapter 8: Nonaqueous Electrolytes
Chapter 9: Electron Work Functions and Volta Potentials
Chapter 10: Structure and Properties of Surface Layers
Chapter 11: Transient Processes
Chapter 12: Electrochemical Research Techniques
PART (II): KINETICS OF ELECTROCHEMICAL REACTIONS
Chapter 13: Multistep Electrode Reactions
Chapter 14: Some Aspects of Electrochemical Kinetics
Chapter 15: Reactions at Nonconsumable Electrodes
Chapter 16: Reactions Involving Metals
PART (III): APPLIED ASPECTS OF ELECTROCHEMISTRY
Chapter 17: Industrial Electrolytic Processes
Chapter 18: Electrochemical Reactors
Chapter 19: Batteries (Electrochemical Power Sources)
Chapter 20: Fuel Cells
Chapter 21: Some Electrochemical Devices
Chapter 22: Corrosion of Metals
Chapter 23: Electrochemical Methods of Analysis
Chapter 24: Electrochemistry and the Environment
PART (IV): SELECTED TOPICS IN ELECTROCHEMISTRY
Chapter 25: Solid-State Electrochemistry
Chapter 26: Conductive Polymers
Chapter 27: Physical Methods for Investigation of Electrode Surfaces
Chapter 28: Electrocatalysis
Chapter 29: Photoelectrochemistry
Chapter 30: Bioelectrochemistry
Chapter 31: Electrokinetic Processes
Chapter 32: Interfaces Between Two Immiscible Electrolyte Solutions
Chapter 33: Various Electrochemical Phenomena
Chapter 34: Main Concepts of Elementary Reaction Act Theory
Chapter 35: Computer Simulation in Electrochemistry
Chapter 36: Nanoelectrochemistry
Chapter 37: Development of Electrochemistry
Cover of Fundamentals of Electrochemistry book
Free Download of Fundamentals of Electrochemistry book
File format: pdf
File size: 6 MB
- For more free books. Visit Category: Free books