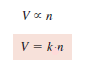Avogadro’s Law: The Volume-Amount Relationship
– In this subject, we will discuss the Avogadro’s Law: The Volume-Amount Relationship
Avogadro’s Law: The Volume-Amount Relationship
– The work of the Italian scientist Amedeo Avogadro complemented the studies of Boyle, Charles, and Gay-Lussac.
– In 1811 Avogadro published a hypothesis stating that at the same temperature and pressure, equal volumes of different gases contain the same number of molecules (or atoms if the gas is monatomic).
– It follows that the volume of any given gas must be proportional to the number of moles of molecules present; that is,
– where n represents the number of moles and k is the proportionality constant.
– The following Equation is the mathematical expression of Avogadro’s law:
- Avogadro’s law states that: “At constant pressure and temperature, the volume of a gas is directly proportional to the number of moles of the gas present”.
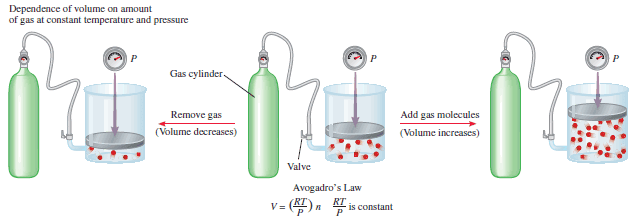
– From the following Figure, we see that:
k = RT/P.
– According to Avogadro’s law, we see that when two gases react with each other, their reacting volumes have a simple ratio to each other.
– If the product is a gas, its volume is related to the volume of the reactants by a simple ratio (a fact demonstrated earlier by Gay-Lussac).
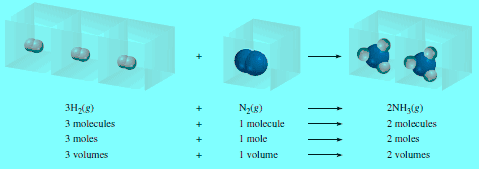
– For example, consider the synthesis of ammonia from molecular hydrogen and molecular nitrogen:
– Because, at the same temperature and pressure, the volumes of gases are directly proportional to the number of moles of the gases present, we can now write:
– The volume ratio of molecular hydrogen to molecular nitrogen is 3:1, and that of ammonia (the product) to the sum of the volumes of molecular hydrogen and molecular nitrogen (the reactants) is 2:4 or 1:2
- Reference: Chemistry / Raymond Chang, Williams College /(10th edition).