General Chemistry
Avogadro’s Number and the Molar Mass of an Element
Avogadro’s Number (NA)
** Atomic mass units provide a relative scale for the masses of the elements.
** But because atoms have such small masses, no usable scale can be devised to weigh them in calibrated units of atomic mass units.
** In any real situation, we deal with macroscopic samples containing enormous numbers of atoms. Therefore, it is convenient to have a special unit to describe a very large number of atoms.
** The idea of a unit to denote a particular number of objects is not new. For example, the pair (2 items), the dozen (12 items), and the gross (144 items) are all familiar units. Chemists measure atoms and molecules in moles.
The mole (mol): is the amount of a substance that contains as many elementary entities (atoms, molecules, or other particles) as there are atom exactly 12 g (or 0.012 kg) of the carbon-12 isotope.
** The actual number of atoms in 12 g of carbon-12 is determined experimentally. This number is called Avogadro’s number (NA), in honor of the Italian scientist Amedeo Avogadro. The currently accepted value is
** Generally, we round Avogadro’s number to 6.022 × 1023. Thus, just as one dozen oranges contains 12 oranges, 1 mole of hydrogen atoms contains 6.022 × 1023 H atoms.
** The following Figure shows samples containing 1 mole each of several common elements.
(One mole each of several common elements. Carbon (black charcoal powder), sulfur (yellow powder), iron (as nails), copper (wires), and mercury (shiny liquid metal).
** The enormity of Avogadro’s number is difficult to imagine. For example, spreading 6.022 × 1023 oranges over the entire surface of Earth would produce a layer 9 mi into space! Because atoms (and molecules) are so tiny, we need a huge number to study them in manageable quantities.
The Molar Mass of an Element
** We have seen that 1 mole of carbon-12 atoms has a mass of exactly 12 g and contains 6.022 × 1023atoms. This mass of carbon-12 is its molar mass (M),
The molar mass (M):is the mass (in grams or kilograms) of 1 mole of units (such as atoms or molecules) of a substance.
** Note that the molar mass of carbon-12 (in grams) is numerically equal to its atomic mass in amu. Likewise, the atomic mass of sodium (Na) is 22.99 amu and its molar mass is 22.99 g; the atomic mass of phosphorus is 30.97 amu and its molar mass is 30.97 g; and so on. If we know the atomic mass of an element, we also know its molar mass.
** Knowing the molar mass and Avogadro’s number, we can calculate the mass of a single atom in grams. For example, we know the molar mass of carbon-12 is 12.00 g and there are 6.022 × 1023 carbon-12 atoms in 1 mole of the substance; therefore, the mass of one carbon-12 atom is given by:
** We can use the preceding result to determine the relationship between atomic mass units and grams. Because the mass of every carbon-12 atom is exactly 12 amu, the number of atomic mass units equivalent to 1 gram is:
This example shows that Avogadro’s number can be used to convert from the atomic mass units to mass in grams and vice versa.
** The following figure shows The relationships between mass (m in grams) of an element and number of moles of an element (n) and between number of moles of an element and number of atoms (N) of an element. μ is the molar mass (g/mol) of the element and NA is Avogadro’s number
** The notions of Avogadro’s number and molar mass enable us to carry out conversions between mass and moles of atoms and between moles and number of atoms (Figure up) . We will employ the following conversion factors in the calculations:
where X represents the symbol of an element. Using the proper conversion factors we can convert one quantity to another.
Solved problems
Problem (1): Zinc (Zn) is a silvery metal that is used in making brass (with copper) and in plating iron to prevent corrosion. How many moles of Zn are there in 45.9 g of Zn?
Strategy:
We are trying to solve for moles of Zn. What conversion factor do we need to convert between grams and moles? Arrange the appropriate conversion factor so that grams cancel and the unit mol is obtained for your answer.
Solution:
The conversion factor needed to convert between grams and moles is the molar mass. In the periodic table (see inside front cover) we see that the molar mass of Zn is 65.39 g. This can be expressed as:
From this equality, we can write the two conversion factors:
The conversion factor on the left is the correct one. Grams will cancel, leaving the unit of mol for the answer. The number of moles of Zn is:
Thus, there is 0.702 mole of Zn in 45.9 g of Zn.
Check:
Because 45.9 g is less than the molar mass of Zn, we expect the result to be less than 1 mole.
Problem (2): Sulfur (S) is a nonmetallic element that is present in coal. When coal is burned, sulfur is converted to sulfur dioxide and eventually to sulfuric acid, which gives rise to the acid rain phenomenon. How many atoms are in 25.1 g of S?
Strategy:
The question asks for atoms of sulfur. We cannot convert directly from grams to atoms of sulfur. What unit do we need to convert grams of sulfur to in order to convert to atoms? What does Avogadro’s number represent?
Solution:
We need two conversions: first from grams to moles and then from moles to number of particles (atoms). The first step is similar to problem (1). Because
the conversion factor is:
Avogadro’s number is the key to the second step. We have:
and the conversion factors are:
The conversion factor on the left is the one we need because it has the number of S atoms in the numerator. We can solve the problem by first calculating the number of moles contained in 25.1 g of S, and then calculating the number of S atoms from the number of moles of S:
We can combine these conversions in one step as follows:
Thus, there are 4.71 × 1023 atoms of S in 25.1 g of S.
Check:
Should 25.1 g S contain fewer than Avogadro’s number of atoms? What mass of S would contain Avogadro’s number of atoms?
Problem (3): Silver (Ag) is a precious metal used mainly in jewelry. What is the mass (in grams) of one Ag atom?
Strategy:
The question asks for the mass of one Ag atom. How many Ag atoms are in 1 mole of Ag and what is the molar mass of Ag?
Solution:
Because 1 mole of Ag atom contains 6.022 × 1023Ag atoms and has a mass of 107.9 g, we can calculate the mass of one Ag atom as follows:
Check:
Because 6.022 × 1023 atoms of Ag have a mass 107.9 g, one atom of Ag should have a significantly smaller mass.
Reference: General Chemistry: The Essential Concepts / Raymond Chang , Jason Overby. (sixth edition).










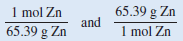











sd
Thank you