Comparison of SN1 and SN2 Reactions
Comparison of SN1 and SN2 Reactions
Let’s compare what we know about the SN1 and SN2 Reactions and reactions, the organize this material into a brief table.
(1) Effect of the Nucleophile on SN1 and SN2 Reactions
– The nucleophile takes part in the slow step (the only step) of the SN2 reaction but not in the slow step of the SN1.
– Therefore, a strong nucleophile promotes the SN2 but not the SN1.
– Weak nucleophiles fail to promote the SN2 reaction; therefore, reactions with weak nucleophiles often go by the SN1 mechanism if the substrate is secondary or tertiary.
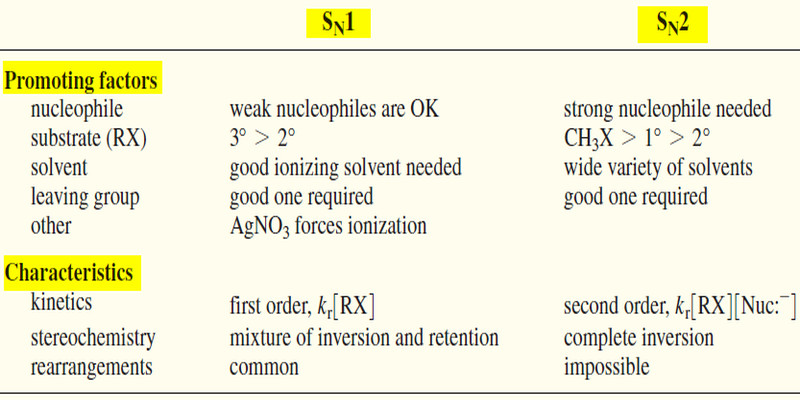
SN1 : Nucleophile strength is unimportant (usually weak).
SN2 : Strong nucleophiles are required
(2) Effect of the Substrate on SN1 and SN2 Reactions
– The structure of the substrate (the alkyl halide) is an important factor in determining which of these substitution mechanisms might operate.
– Most methyl halides and primary halides are poor substrates for SN1 substitutions because they cannot easily ionize to high-energy methyl and primary carbocations.
– They are relatively unhindered, however, so they make good SN2 substrates.
– Tertiary halides are too hindered to undergo SN2 displacement, but they can ionize to form tertiary carbocations.
– Tertiary halides undergo substitution exclusively through the SN1 mechanism.
– Secondary halides can undergo substitution by either mechanism, depending on the conditions.
SN1 substrates : 3° > 2° (1° and CH3X are unlikely)
SN2 substrates : CH3X > 1° < 2° (3° is unsuitable)
– If silver nitrate (AgNO3) is added to an alkyl halide in a good ionizing solvent, the silver ion removes the halide ion to give a carbocation.
– This technique can force some unlikely ionizations, often giving interesting rearrangements.
(3) Effect of the Solvent on SN1 and SN2 Reactions
– The slow step of the SN1 reaction involves formation of two ions.
– Solvation of these ions is crucial to stabilizing them and lowering the activation energy for their formation.
– Very polar ionizing solvents such as water and alcohols are needed for the SN1.
– The solvent may be heated to reflux (boiling) to provide the energy needed for ionization.
– Less charge separation is generated in the transition state of the SN2 reaction.
– Strong solvation may weaken the strength of the nucleophile because of the energy needed to strip off the solvent molecules.
– Thus, the SN2 reaction often goes faster in less polar solvents if the nucleophile will dissolve.
– Polar aprotic solvents may enhance the strength of weak nucleophiles.
SN1 : Good ionizing solvent required.
SN2 : May go faster in a less polar solvent.
(4) Kinetics of SN1 and SN2 Reactions
– The rate of the SN1 reaction is proportional to the concentration of the alkyl halide but not the concentration of the nucleophile. It follows a first-order rate equation.
– The rate of the SN2 reaction is proportional to the concentrations of both the alkyl halide [R-X] and the nucleophile [Nuc:– ]. It follows a second-order rate equation.
SN1 rate = kr [R-X]
SN2 rate = kr [R-X] [Nuc:– ]
(5) Stereochemistry of SN1 and SN2 Reactions
– The SN1 reaction involves a flat carbocation intermediate that can be attacked from either face. Therefore, the SN1 usually gives a mixture of inversion and retention of configuration.
– The SN2 reaction takes place through a back-side attack, which inverts the stereochemistry of the carbon atom. Complete inversion of configuration is the result.
SN1 stereochemistry : Mixture of retention and inversion; racemization.
SN2 stereochemistry : Complete inversion.
(6) Rearrangements on SN1 and SN2 Reactions
– The SN1 reaction involves a carbocation intermediate. This intermediate can rearrange, usually by a hydride shift or an alkyl shift, to give a more stable carbocation.
– The SN2 reaction takes place in one step with no intermediates. No rearrangement is possible in the SN2 reaction.
SN1: Rearrangements are common.
SN2: Rearrangements are impossible.