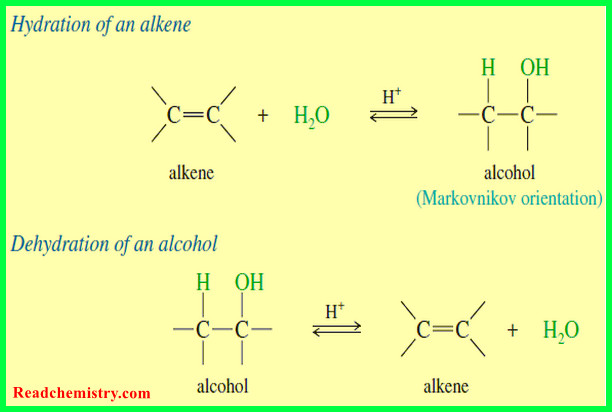
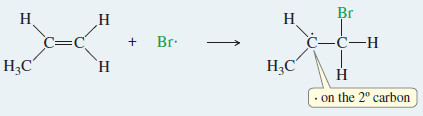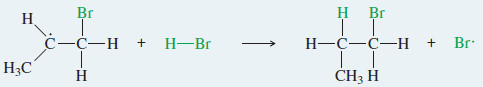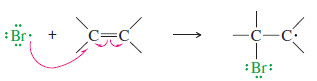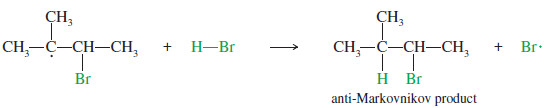Addition of Hydrogen Halides to Alkenes: Markovnikov’s Rule
Addition of Hydrogen Halides to Alkenes : Markovnikov’s Rule
Orientation of Addition: Markovnikov’s Rule
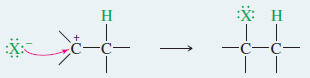
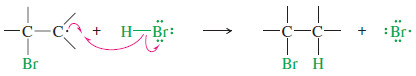
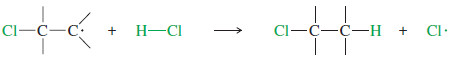
– The simple mechanism shown for addition of HBr to but-2-ene applies to a large number of electrophilic additions.
– We can use this mechanism to predict the outcome of some fairly complicated reactions.
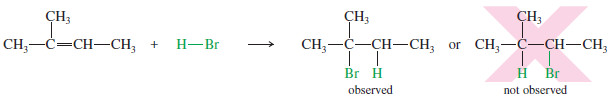
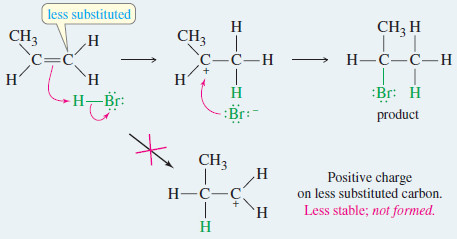
– For example, the addition of HBr to 2-methylbut-2- ene could lead to either of two products, yet only one is observed.
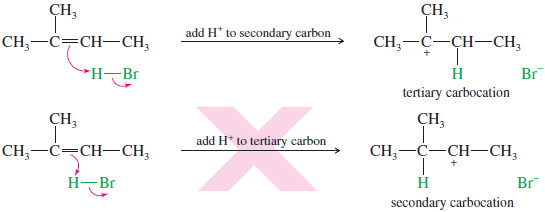
– The first step is protonation of the double bond.
– If the proton adds to the secondary carbon, the product will be different from the one formed if the proton adds to the tertiary carbon.
– When the proton adds to the secondary carbon, a tertiary carbocation results.
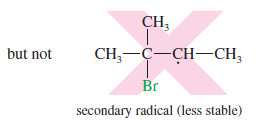
– When the proton adds to the tertiary carbon atom, a secondary carbocation results.
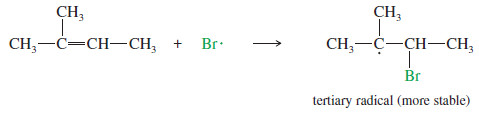
– The tertiary carbocation is more stable, so the first reaction is favored.
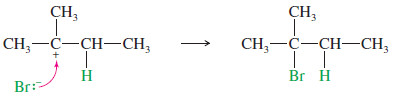
– The second half of the mechanism produces the final product of the addition of HBr to 2-methylbut-2-ene.
– Note that protonation of one carbon atom of a double bond gives a carbocation on the carbon atom that was not protonated.
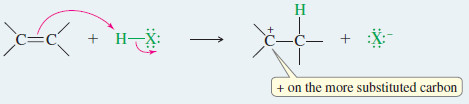
– Therefore, the proton adds to the end of the double bond that is less substituted to give the more substituted carbocation (the more stable carbocation)
Mechanism: Ionic Addition of HX to an Alkene
Step 1: Protonation of the pi bond forms a carbocation.
Step 2: Attack by the halide ion gives the addition product.
Example:
– The ionic addition of HBr to propene shows protonation of the less substituted carbon to give the more substituted carbocation.
– Reaction with bromide ion completes the addition
– There are many examples of reactions where the proton adds to the less substituted carbon atom of the double bond in order to produce the more substituted carbocation.
– The addition of HBr (and other hydrogen halides) is said to be regioselective because in each case, one of the two possible orientations of addition results preferentially over the other.
Markovnikov’s Rule statement
– A Russian chemist, Vladimir Markovnikov, first showed the orientation of addition of HBr to alkenes in 1869.
– Markovnikov stated:
Markovnikov’s rule
(The addition of a proton acid to the double bond of an alkene results in a product with the acid proton bonded to the carbon atom that already holds the greater number of hydrogen atoms)
– This is the original statement of Markovnikov’s rule.
– Reactions that follow this rule are said to follow Markovnikov orientation and give the Markovnikov product.
– We are often interested in adding electrophiles other than proton acids to the double bonds of alkenes.
– Markovnikov’s rule can be extended to include a wide variety of other additions, based on the addition of the electrophile in such a way as to produce the most stable carbocation.
Markovnikov’s rule (extended):
(In an electrophilic addition to an alkene, the electrophile adds in such a way as to generate the most stable intermediate.)
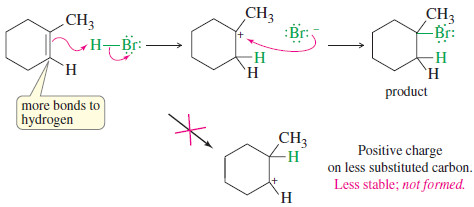
– The following figure shows how HBr adds to 1-methylcyclohexene to give the product with an additional hydrogen bonded to the carbon that already had the most bonds to hydrogen (one) in the alkene.
– Note that this orientation results from addition of the proton in the way that generates the more stable carbocation.
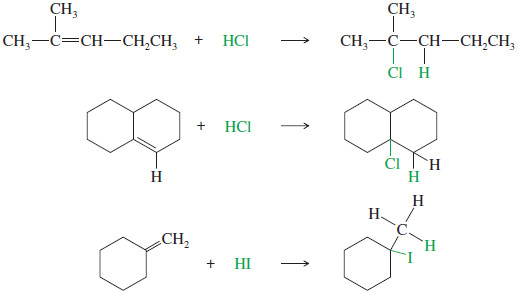
– Like HBr, both HCl and HI add to the double bonds of alkenes, and they also follow Markovnikov’s rule; for example
(2) Free-Radical Addition of HBr: Anti-Markovnikov Addition
– In 1933, M. S. Kharasch and F. W. Mayo found that some additions of HBr (but not HCl or HI) to alkenes gave products that were opposite to those expected from Markovnikov’s rule.
– These anti-Markovnikov reactions were most likely when the reagents or solvents came from old supplies that had accumulated peroxides from exposure to the air.
– Peroxides give rise to free radicals that initiate the addition, causing it to occur by a radical mechanism.
– The oxygen–oxygen bond in peroxides is rather weak, so it can break to give two alkoxy radicals
ΔH° = +150 kJ (+36 kcal
– Alkoxy radicals (R-O.)initiate the anti-Markovnikov addition of HBr.
– The mechanism of this free-radical chain reaction is shown in the following Mechanism
Mechanism: Free-Radical Addition of HBr to Alkenes
Initiation: Formation of radicals
Propagation: A radical reacts to generate another radical.
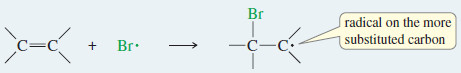
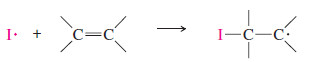
Step 1: A bromine radical adds to the double bond to generate an alkyl radical on the more substituted carbon atom.
Step 2: The alkyl radical abstracts a hydrogen atom from HBr to generate the product and a bromine radical.
– The bromine radical generated in Step 2 goes on to react with another molecule of alkene in Step 1, continuing the chain.
Example: Free-radical addition of HBr to propene.
Initiation: Radicals are formed.
Propagation: A radical reacts to generate another radical.
Step 1: A bromine radical adds to the double bond to generate an alkyl radical on the secondary carbon atom.
Step 2: The alkyl radical abstracts a hydrogen atom from HBr to generate the product and a bromine radical.
– The bromine radical generated in Step (2) goes on to react in Step 1, continuing the chain.
– Let’s consider the individual steps. In the initiation step, free radicals generated from the peroxide react with HBr to form bromine radicals
ΔH° = –63 kJ (–15 kcal)
– The bromine radical lacks an octet of electrons in its valence shell, making it electrondeficient and electrophilic.
– It adds to a double bond, forming a new free radical with the odd electron on a carbon atom
ΔH= –12 kJ (–3 kcal)
– This free radical reacts with an HBr molecule to form a C – H bond and generate another bromine radical.
ΔH= –25 kJ (–6 kcal)
– The regenerated bromine radical reacts with another molecule of the alkene, continuing the chain reaction.
– Both of the propagation steps are moderately exothermic, allowing them to proceed faster than the termination steps.
– Note that each propagation step starts with one free radical and ends with another free radical.
– The number of free radicals is constant, until the reactants are consumed, and free radicals come together and terminate the chain reaction
(3) Radical Addition of HBr to Unsymmetrical Alkenes
– Now we must explain the anti- Markovnikov orientation found in the products of the peroxide catalyzed reaction.
– With an unsymmetrical alkene like 2-methylbut-2-ene, adding the bromine radical to the secondary end of the double bond forms a tertiary radical
– As we saw in the protonation of an alkene, the electrophile (in this case, Br . ) adds to the less substituted end of the double bond, and the unpaired electron appears on the more substituted carbon to give the more stable free radical.
– This intermediate reacts with HBr to give the anti Markovnikov product, in which H has added to the more substituted end of the double bond: the end that started with fewer hydrogens.
– Note that both mechanisms for the addition of HBr to an alkene (with and without peroxides) follow our extended statement of Markovnikov’s rule: In both cases, the electrophile adds to the less substituted end of the double bond to give the more stable intermediate, either a carbocation or a free radical.
– In the ionic reaction, the electrophile is H+. In the peroxide-catalyzed free-radical reaction Br . is the electrophile.
– Many students wonder why the reaction with Markovnikov orientation does not take place in the presence of peroxides, together with the free-radical chain reaction.
– It actually does take place, but the peroxide-catalyzed reaction is faster.
– If just a tiny bit of peroxide is present, a mixture of Markovnikov and anti-Markovnikov products results.
– If an appreciable amount of peroxide is present, the radical chain reaction is so much faster than the uncatalyzed ionic reaction that only the anti-Markovnikov product is observed.
– The reversal of orientation in the presence of peroxides is called the peroxide effect. It occurs only with the addition of HBr to alkenes.
– The peroxide effect is not seen with HCl because the reaction of an alkyl radical with HCl is strongly endothermic.
ΔH° = +42 kJ (+10 kcal)
– Similarly, the peroxide effect is not observed with HI because the reaction of an iodine atom with an alkene is strongly endothermic.
– Only HBr has just the right reactivity for each step of the free-radical chain reaction to take place
ΔH° = +42 kJ (+10 kcal)
Solved Proplem
Show how you would accomplish the following synthetic conversions.
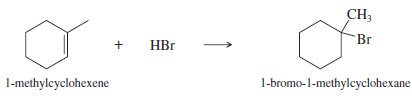
(a) Convert 1-methylcyclohexene to 1-bromo-1-methylcyclohexane.
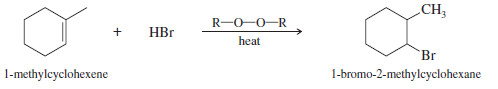
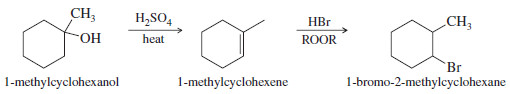
(b) Convert 1-methylcyclohexanol to 1-bromo-2-methylcyclohexane
Solution
(a)
– This synthesis requires the addition of HBr to an alkene with Markovnikov orientation.
-Ionic addition of HBr gives the correct product.
(b)
– This synthesis requires the conversion of an alcohol to an alkyl bromide with the bromine atom at the neighboring carbon atom.
– This is the anti-Markovnikov product, which could be formed by the radical-catalyzed addition of HBr to 1-methylcyclohexene
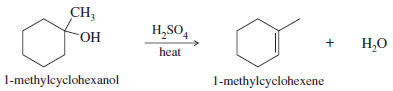
1-Methylcyclohexene is easily synthesized by the dehydration of 1-methylcyclohexanol. The most substituted alkene is the desired product
The two-step synthesis is summarized as follows: