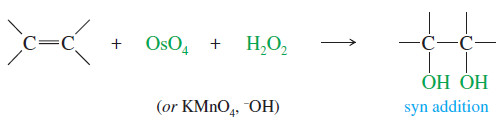Syn Dihydroxylation of Alkenes
Syn Dihydroxylation of Alkenes
– Converting an alkene to a glycol requires adding a hydroxyl group to each end of the double bond. This addition is called Dihydroxylation of Alkenes or dihydroxylation (or hydroxylation) of the double bond.
– We have seen that epoxidation of an alkene, followed by acidic hydrolysis, gives anti dihydroxylation of the double bond.
– Reagents are also available for the dihydroxylation of alkenes with syn stereochemistry.
– The two most common reagents for this purpose are osmium tetroxide and potassium permanganate.
(A) Osmium Tetroxide Dihydroxylation
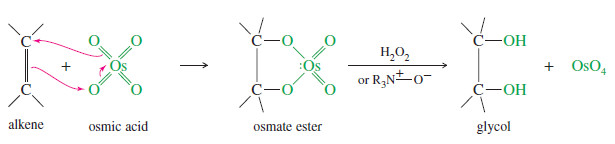
– Osmium tetroxide (OsO4 , sometimes called osmic acid) reacts with alkenes in a concerted step to form a cyclic osmate ester.
– Oxidizing agents such as hydrogen peroxide (H2O2) or tertiary amine oxides (R3N+ – O–) are used to hydrolyze the osmate ester and reoxidize osmium to osmium tetroxide.
– The regenerated osmium tetroxide catalyst continues to hydroxylate more molecules of the alkene.
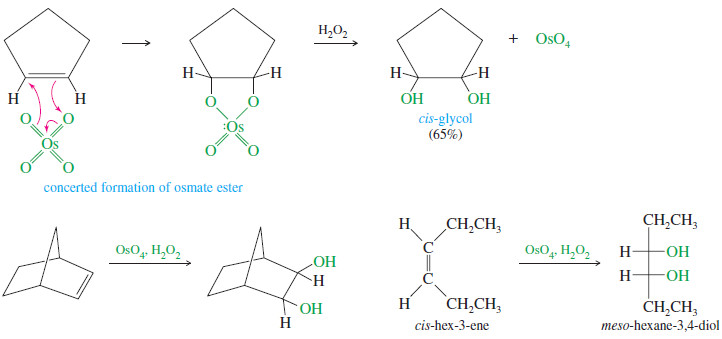
– Because the two carbon–oxygen bonds are formed simultaneously with the cyclic osmate ester, the oxygen atoms add to the same face of the double bond; that is, they add with syn stereochemistry.
– The following reactions show the use of OsO4 and H2O2 for the syn dihydroxylation of alkenes.
(B) Permanganate Dihydroxylation
– Osmium tetroxide is expensive, highly toxic, and volatile.
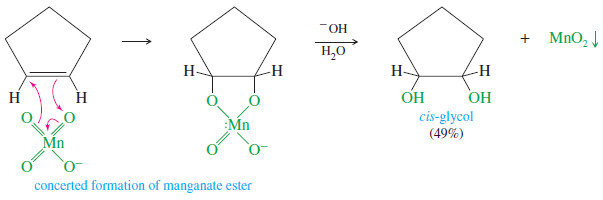
– A cold, dilute solution of potassium permanganate (KMnO4) also hydroxylates alkenes with syn stereochemistry, with slightly reduced yields in most cases.
– Like osmium tetroxide, permanganate adds to the alkene double bond to form a cyclic ester: a manganate ester in this case.
– The basic solution hydrolyzes the manganate ester, liberating the glycol and producing a brown precipitate of manganese dioxide, MnO2
– In addition to its synthetic value, the permanganate oxidation of alkenes provides a simple chemical test for the presence of an alkene.
– When an alkene is added to a clear, deep purple aqueous solution of potassium permanganate, the solution loses its purple color and becomes the murky, opaque brown color of MnO2. (Although there are other functional groups that decolorize permanganate, few do it as quickly as alkenes.)
(c) Choosing a Reagent
– To dihydroxylate an alkene with syn stereochemistry, which is the better reagent: osmium tetroxide or potassium permanganate? Osmium tetroxide gives better yields, but permanganate is cheaper and safer to use. The answer depends on the circumstances.
– If the starting material is only 2 mg of a compound 15 steps along in a difficult synthesis, we use osmium tetroxide.
– The better yield is crucial because the starting material is precious and expensive, and little osmic acid is needed.
– If the dihydroxylation is the first step in a synthesis and involves 5 kg of the starting material, we use potassium permanganate.
– The cost of buying enough osmium tetroxide would be prohibitive, and dealing with such a large amount of a volatile, toxic reagent would be inconvenient.
– On such a large scale, we can accept the lower yield of the permanganate oxidation.