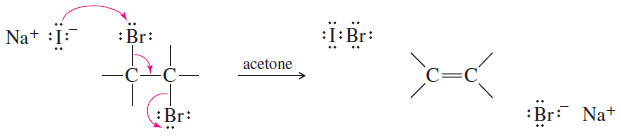Alkene Synthesis by Elimination of Alkyl Halides
Alkene Synthesis by Elimination of Alkyl Halides
– Dehydrohalogenation is the elimination of a hydrogen and a halogen from an alkyl halide to form an alkene.
– dehydrohalogenation can take place by the E1 and E2 mechanisms.
– The second-order elimination (E2) is usually better for synthetic purposes because the E1 has more competing reactions.
(1) Dehydrohalogenation by the E2 Mechanism
– Second-order elimination is a reliable synthetic reaction, especially if the alkyl halide is a poor SN2 substrate.
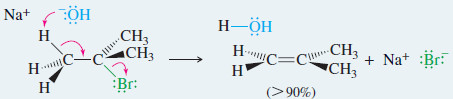
– E2 dehydrohalogenation takes place in one step, in which a strong base abstracts a proton from one carbon atom as the leaving group leaves the adjacent carbon
MECHANISM :Dehydrohalogenation by the E2 Mechanism
– E2 elimination takes place by a concerted one-step reaction.
– A strong base abstracts a proton on a carbon next to the one bearing a halogen.
– The leaving group (halide) leaves simultaneously.
EXAMPLE: E2 elimination of tert-butyl bromide with sodium hydroxide
– The E2 dehydrohalogenation gives excellent yields with bulky secondary and tertiary alkyl halides, such as tert-butyl bromide in the preceding example.
– A strong base forces second-order elimination (E2) by abstracting a proton.
– The molecule’s bulkiness hinders second-order substitution SN2 and a relatively pure elimination product results.
– Tertiary halides are the best E2 substrates because they are prone to elimination and cannot undergo SN2 substitution.
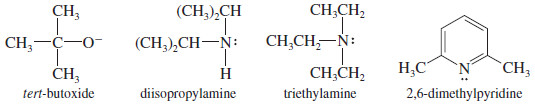
Use of a Bulky Base
– If the substrate is prone to substitution, a bulky base can minimize the amount of substitution.
– Large alkyl groups on a bulky base hinder its approach to attack a carbon atom (substitution), yet it can easily abstract a proton (elimination).
– Some of the bulky strong bases commonly used for elimination are tert-butoxide ion, diisopropylamine, triethylamine, and 2,6-dimethylpyridine
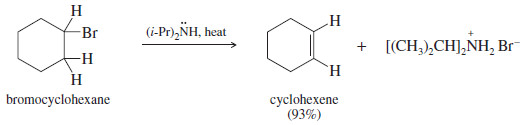
– The dehydrohalogenation of bromocyclohexane illustrates the use of a bulky base for elimination.
– Bromocyclohexane, a secondary alkyl halide, can undergo both substitution and elimination.
– Elimination (E2) is favored over substitution SN2 by using a bulky base such as diisopropylamine.
– Diisopropylamine is too bulky to be a good nucleophile, but it acts as a strong base to abstract a proton
Formation of the Hofmann Product
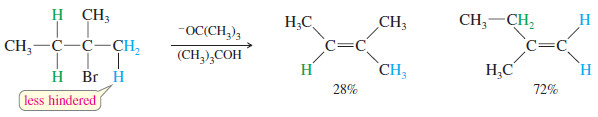
– Bulky bases can also accomplish dehydrohalogenations that do not follow the Zaitsev rule.
– Steric hindrance often prevents a bulky base from abstracting the proton that leads to the most highly substituted alkene.
– In these cases, it abstracts a less hindered proton, often the one that leads to formation of the least highly substituted product, called the Hofmann product.
– The following reaction gives mostly the Zaitsev product with the relatively unhindered ethoxide ion, but mostly the Hofmann product with the bulky tert-butoxide ion.
(2) Stereospecific E2 Reactions
– Like the SN2 reaction, the E2 is stereospecific: Different stereoisomers of the reactant give different stereoisomers of the product.
– The E2 is stereospecific because it normally goes through an anti and coplanar transition state.
– The products are alkenes, and different diastereomers of starting materials commonly give different diastereomers of alkenes.
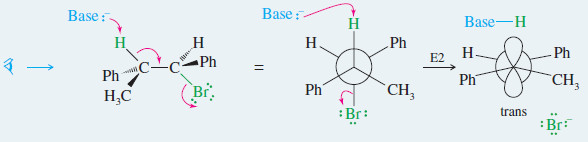
– In Problem 6-38, you showed why the E2 elimination of one diastereomer of 1-bromo-1,2 diphenylpropane gives only the trans isomer of the alkene product
If we make a model and look at this reaction from the left end of the molecule, the anti
and coplanar arrangement of the H and Br is apparent.
MECHANISM: Stereochemistry of the E2 Reaction
– Most E2 reactions go through an anti-coplanar transition state.
– This geometry is most apparent if we view the reaction sighting down the carbon–carbon bond between the hydrogen and leaving group.
– Viewed from the left:
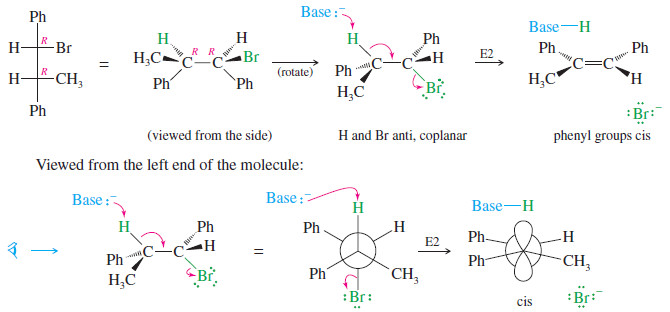
– The following reaction shows how the anti-coplanar elimination of the other diastereomer (R,R) gives only the cis isomer of the product.
In effect, the two different diastereomers of the reactant give two different diastereomers of the product: a stereospecific result.
(3) E2 Reactions in Cyclohexane Systems
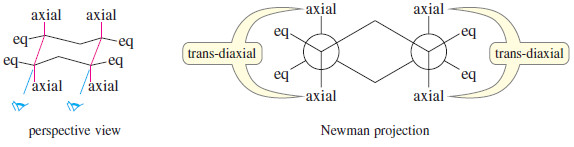
– Nearly all cyclohexanes are most stable in chair conformations.
– In the chair, all the carbon–carbon bonds are staggered, and any two adjacent carbon atoms have axial bonds in an anti-coplanar conformation, ideally oriented for the E2 reaction. (As drawn in the following figure, the axial bonds are vertical.)
– On any two adjacent carbon atoms, one has its axial bond pointing up and the other has its axial bond pointing down.
– These two bonds are trans to each other, and we refer to their geometry as trans-diaxial.
– An E2 elimination can take place on this chair conformation only if the proton and the leaving group can get into a trans-diaxial arrangement.
– The following figure shows the E2 dehydrohalogenation of bromocyclohexane.
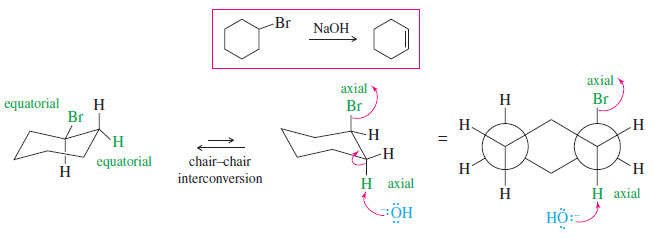
– The molecule must flip into the chair conformation with the bromine atom axial before elimination can occur.
(You should make models of the structures in the following examples and problems so you can follow along more easily.)
Solved problem
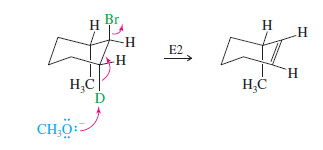
Explain why the following deuterated 1-bromo-2-methylcyclohexane undergoes dehydrohalogenation by the E2 mechanism, to give only the indicated product. Two other alkenes are not observed.
Solution:
In an E2 elimination, the hydrogen atom and the leaving group must have a trans-diaxial relationship.
In this compound, only one hydrogen atom—the deuterium—is trans to the bromine atom. When the bromine atom is axial, the adjacent deuterium is also axial, providing a transdiaxial arrangement.
(4) Debromination of Vicinal Dibromides
– Vicinal dibromides (two bromines on adjacent carbon atoms) are converted to alkenes by reduction with iodide ion in acetone.
– This debromination is rarely an important synthetic reaction, because the most likely origin of a vicinal dibromide is from bromination of an alkene.
– We discuss this reaction with dehydrohalogenation because the mechanisms are similar.
– Debromination is formally a reduction because a molecule of Br2 (an oxidizing agent) is removed.
– The reaction with iodide takes place by the E2 mechanism, with the same geometric constraints as the E2 dehydrohalogenation. Elimination usually takes place through an anti-coplanar arrangement, as shown in the following Mechanism.
– Acetone serves as a convenient solvent that dissolves most alkyl halides and sodium iodide.
MECHANISM: E2 Debromination of a Vicinal Dibromide
– E2 debromination takes place by a concerted, stereospecific mechanism.
– Iodide ion removes one bromine atom, and the other bromine leaves as bromide ion.
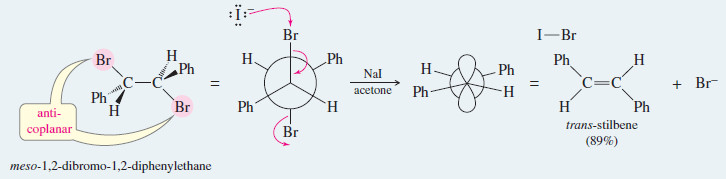
Use your models to show that only the trans isomer of stilbene is formed in this example by elimination through the anti-coplanar transition state.
Solved problem
Show that the dehalogenation of 2,3-dibromobutane by iodide ion is stereospecific by showing that the two diastereomers of the starting material give different diastereomers of the product.
Solution:
– Rotating meso-2,3-dibromobutane into a conformation where the bromine atoms are anti and coplanar, we find that the product will be trans-but-2-ene.
– A similar conformation of either enantiomer of the (±) diastereomer shows that the product will be cis-but-2-ene. (Hint: Your models will be helpful.)
(5) Dehydrohalogenation by the E1 Mechanism
– First-order dehydrohalogenation usually takes place in a good ionizing solvent (such as an alcohol or water), without a strong nucleophile or base to force second-order kinetics.
– The substrate is usually a secondary or tertiary alkyl halide.
– First-order elimination requires ionization to form a carbocation, which loses a proton to a weak base(usually the solvent).
– E1 dehydrohalogenation is generally accompanied by SN1 substitution, because the nucleophilic solvent can also attack the carbocation directly to form the substitution product.
Elimination by the E1 mechanism
Like all reactions involving carbocation intermediates, E1 dehydrohalogenations are prone to rearrangement.