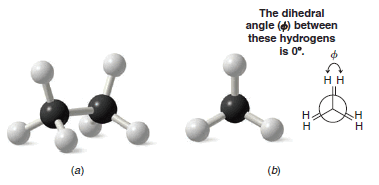Sigma Bond and Bond Rotation
– In this subject, we will discuss the Sigma Bond and Bond Rotation
– Two groups bonded by only a single bond can undergo rotation about that bond for each other:
– The temporary molecular shapes that result from such a rotation are called conformations of the molecule.
– Each possible structure is called a conformer.
– An analysis of the energy changes that occur as a molecule undergoes rotations about single bonds is called a conformational analysis.
Newman Projection and How To Draw Them
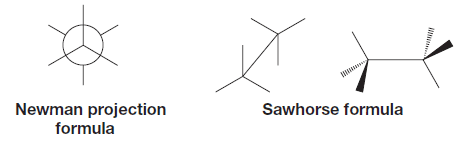
– When we do conformational analysis, we will find that certain types of structural formulas are especially convenient to use.
– One of these types is called a Newman projection formula and another type is a sawhorse formula.
– Sawhorse formulas are much like dash–wedge three-dimensional formulas we have used so far.
– In conformational analyses, we will make substantial use of Newman projections.
How to write a Newman projection formula?
– We imagine ourselves taking a view from one atom (usually a carbon) directly along a selected bond axis to the next atom (also usually a carbon atom).
– The front carbon and its other bonds are represented as:
– The back carbon and its bonds are represented as:
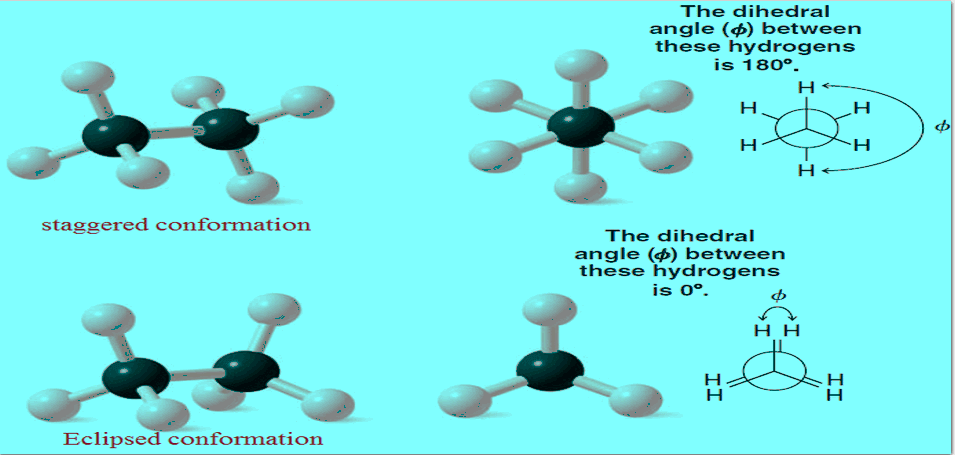
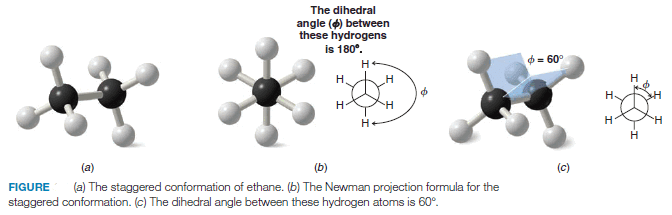
– In Fig (a), and (b) we show ball-and-stick models and a Newman projection formula for the staggered conformation of ethane.
– The staggered conformation of a molecule is that conformation where the dihedral angle between the bonds at each of the carbon-carbon bonds is 180o and where atoms or groups bonded to carbons at each end of a carbon-carbon bond are as far apart as possible.
– The 180o dihedral angle in the staggered conformation of ethane is indicated in Fig b.
– The eclipsed conformation of ethane is shown in Fig blow using ball-and-stick models and a Newman projection.
– In an eclipsed conformation the atoms bonded to carbons at each end of a carbon-carbon bond are directly opposed to one another. The dihedral angle between them is 0o.
Figure (a): The eclipsed conformation of ethane.
Figure (b): The Newman projection formula for the eclipsed conformation
How To Do a Conformational Analysis
– Now let us consider a conformational analysis of ethane:
– Clearly, infinitesimally small changes in the dihedral angle between C-H bonds at each end of ethane could lead to an infinite number of conformations, including, of course, the staggered and eclipsed conformations.
– These different conformations are not all of equal stability, however, and it is known that the staggered conformation of ethane is the most stable conformation (i.e., it is the conformation of lowest potential energy).
– The explanation for greater stability of the staggered conformation relates mainly to steric repulsion between bonding pairs of electrons.
– In the eclipsed conformation electron clouds from the C-H bonds are closer and repel one another.
– The staggered conformation allows the maximum possible separation of the electron pairs in the C-H bonds.
– In addition, there is a phenomenon called hyperconjugation that involves favorable overlap between filled and unfilled sigma orbitals in the staggered conformation.
– Hyperconjugation helps to stabilize the staggered conformation.
– The more important factor, however, is the minimization of steric repulsions in the staggered form.
The energy difference between the conformations of ethane
– It can be represented graphically in a potential energy diagram, as shown in Figure.
– In ethane, the energy difference between the staggered and eclipsed conformations is about 12 kJ mol-1.
– This small barrier to rotation is called the torsional barrier of the single bond.
– Because of the torsional barrier, some molecules will wag back and forth with their atoms in staggered or nearly staggered conformations, while others with slightly more energy will rotate through an eclipsed conformation to another staggered conformation.
– At any given moment, unless the temperature is extremely low (-250 oC), most ethane molecules will have enough energy to undergo bond rotation from one conformation to another.
What does all this mean about ethane?
– We can answer this question in two different ways:
– If we consider a single molecule of ethane, we can say, for example, that it will spend most of its time in the lowest energy, staggered conformation, or in a conformation very close to being staggered.
– Many times every second, however, it will acquire enough energy through collisions with other molecules to surmount the torsional barrier and it will rotate through an eclipsed conformation.
– If we speak in terms of a large number of ethane molecules (a more realistic situation), we can say that at any given moment most of the molecules will be in staggered or nearly staggered conformations.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.