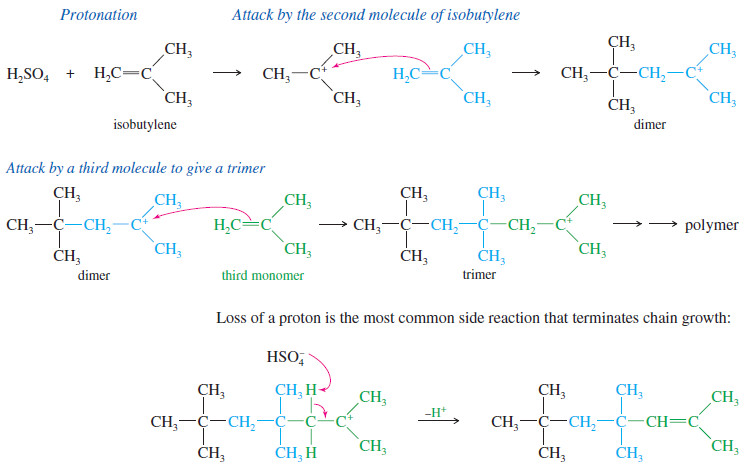
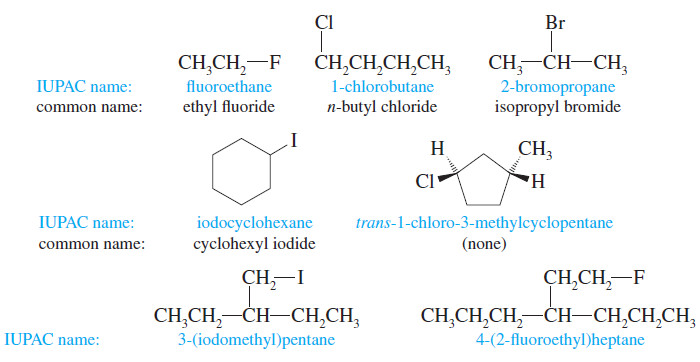
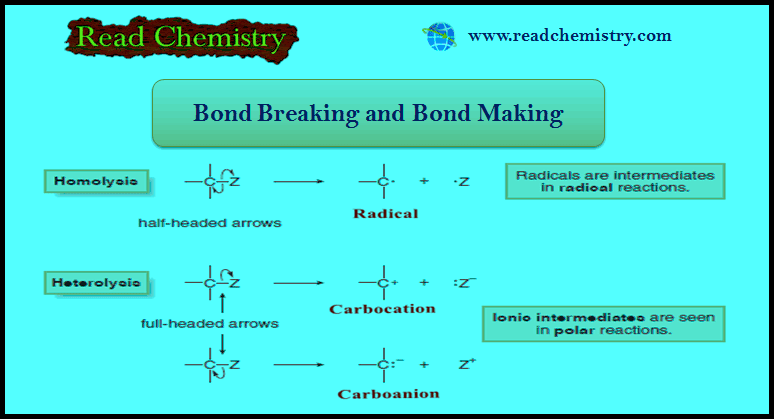
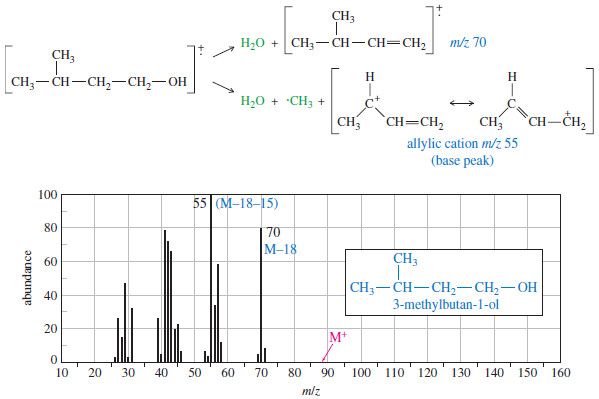
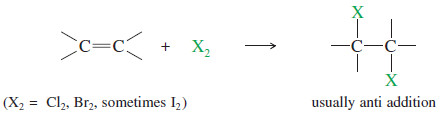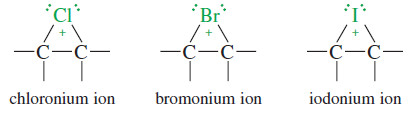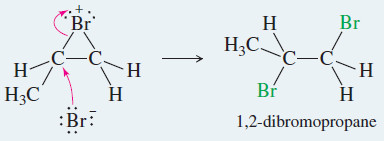Addition of Halogens to Alkenes
Addition of Halogens to Alkenes
– Addition of Halogens to Alkenes gives vicinal dihalides.
(A) Mechanism of Halogen Addition
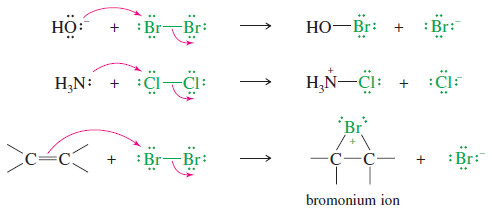
– A halogen molecule (Br2, Cl2, or I2) is electrophilic; a nucleophile can react with a halogen, displacing a halide ion:
– In this example, the nucleophile attacks the electrophilic nucleus of one bromine atom, and the other bromine serves as the leaving group, departing as bromide ion.
– Many reactions fit this general pattern; for example:
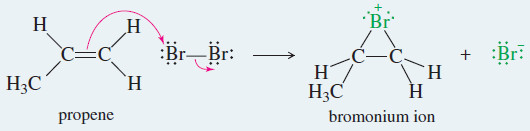
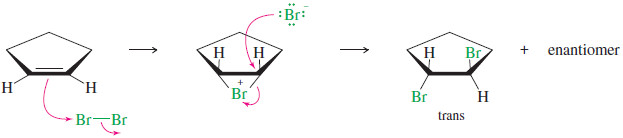
– In the last reaction, the pi electrons of an alkene attack the bromine molecule, expelling bromide ion.
– A bromonium ion results, containing a three-membered ring with a positive charge on the bromine atom.
– This bromonium ion is similar in structure to the mercurinium ion.
– Similar reactions with other halogens form other halonium ions.
– The structures of a chloronium ion, a bromonium ion, and an iodonium ion are shown next.
Examples
– Unlike a normal carbocation, all the atoms in a halonium ion have filled octets.
– The three-membered ring has considerable ring strain, however, which, combined with a positive charge on an electronegative halogen atom, makes the halonium ion strongly electrophilic.
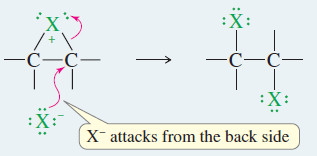
– Attack by a nucleophile, such as a halide ion, opens the halonium ion to give a stable product.
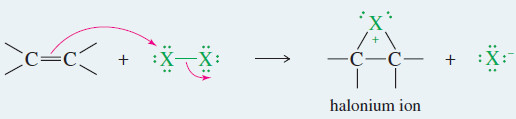
mechanism: Addition of Halogens to Alkenes
Step 1: Electrophilic attack forms a halonium ion.
Step 2: The halide ion opens the halonium ion.
Example: Addition of Br2 to propene.
Step 1: Electrophilic attack forms a bromonium ion.
Step 2: Bromide ion opens the bromonium ion.
– Chlorine and bromine commonly add to alkenes by the halonium ion mechanism.
– Iodination is used less frequently because diiodide products decompose easily.
– Any solvents used must be inert to the halogens; methylene chloride (CH2Cl2) chloroform (CHCl3) and carbon tetrachloride (CCl4) are the most frequent choices.
– The addition of bromine has been used as a simple chemical test for the presence of olefinic double bonds.
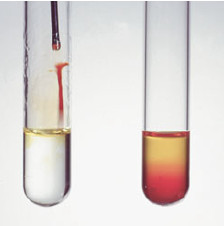
– A solution of bromine in carbon tetrachloride is a clear, deep red color.
– When this red solution is added to an alkene, the red bromine color disappears (we say it is “decolorized”), and the solution becomes clear and colorless (Although there are other functional groups that decolorize bromine, few do it as quickly as alkenes.)
– When a solution of bromine (red-brown) is added to cyclohexene, the bromine color quickly disappears because bromine adds across the double bond.
– When bromine is added to cyclohexane (at right), the color persists because no reaction occurs.
(B) Stereochemistry of Halogen Addition
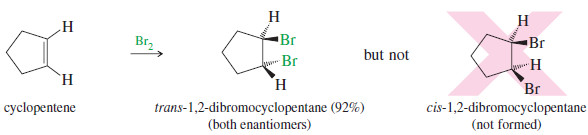
– The addition of bromine to cyclopentene is a stereospecific anti addition.
– Anti stereochemistry results from the bromonium ion mechanism.
– When a nucleophile attacks a halonium ion, it must do so from the back side, in a manner similar to the SN2 displacement.
– This back-side attack assures anti stereochemistry of addition.
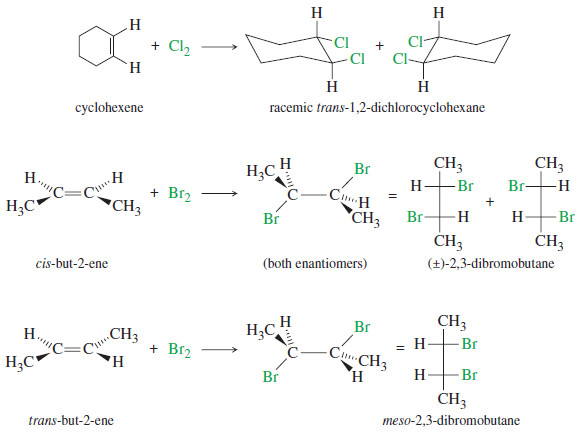
– Halogen addition is another example of a stereospecific reaction, in which different stereoisomers of the starting material give different stereoisomers of the product.
– The following Figure shows additional examples of the anti addition of halogens to alkenes.