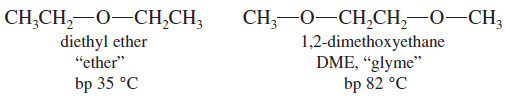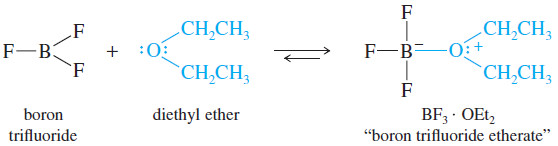Physical Properties of Ethers
– In this topic, we will discuss The Physical Properties of Ethers.
Introduction to Ethers
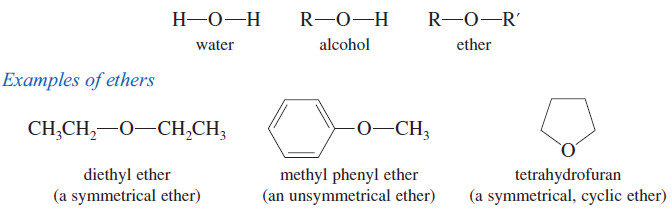
– Ethers are compounds of formula R-O-R’ where R and R’ may be alkyl groups or aryl (benzene ring) groups.
– Like alcohols, ethers are related to water, with alkyl groups replacing the hydrogen atoms.
– In an alcohol, one hydrogen atom of water is replaced by an alkyl group.
– In an ether, both hydrogens are replaced by alkyl groups.
– The two alkyl groups are the same in a symmetrical ether and different in an unsymmetrical ether.
– As with other functional groups, we will discuss how ethers are formed and how they react.
– Ethers (other than epoxides) are relatively unreactive, however, and they are not frequently used as synthetic intermediates.
– Because they are stable with many types of reagents, ethers are commonly used as solvents for organic reactions.
– The most important commercial ether is diethyl ether, often called “ethyl ether,” or simply “ether.”
– Ether is a good solvent for reactions and extractions, and it is used as a volatile starting fluid for diesel and gasoline engines.
– Ether was used as a surgical anesthetic for over a hundred years (starting in 1842), but it is highly flammable, and patients often vomited as they regained consciousness.
– Several compounds that are less flammable and more easily tolerated are now in use, including nitrous oxide and (N2O) and halothane (CF3-CHClBr)
Physical Properties of Ethers
(1) Structure and Polarity of Ethers
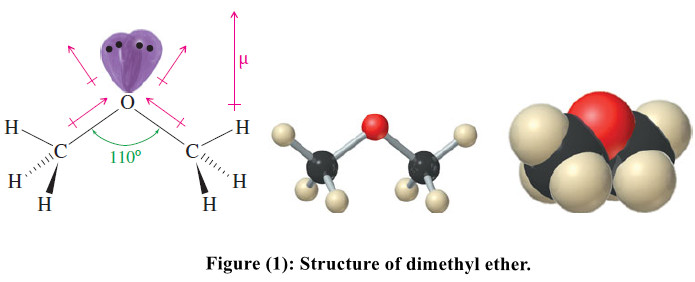
– Like water, ethers have a bent structure, with an sp3 hybrid oxygen atom giving a nearly tetrahedral bond angle.
– In water, the nonbonding electrons compress the H-O-H bond angle to 104.5°, but in a typical ether, the bulk of the alkyl groups enlarges the bond angle.
– Figure(1) shows the structure of dimethyl ether, with a tetrahedral bond angle of 110°.
– Although ethers lack the polar hydroxyl group of alcohols, they are still strongly polar compounds.
– The dipole moment of an ether is the vector sum of two polar C-O bonds, with a substantial contribution from the two lone pairs of electrons.
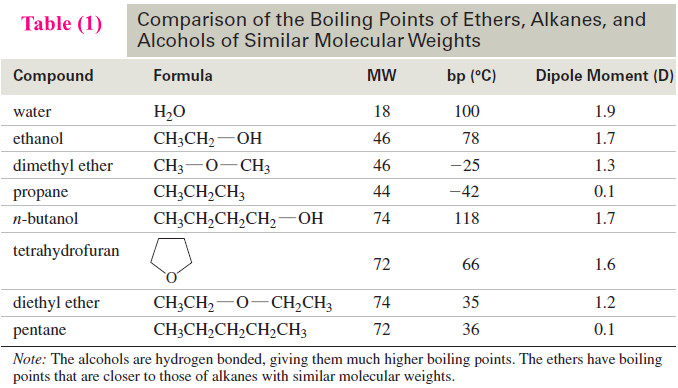
– Table (1) compares the dipole moments of dimethyl ether, diethyl ether, and tetrahydrofuran (THF) with those of alkanes and alcohols of similar molecular weights.
– An ether such as THF provides a strongly polar solvent without the reactivity of a hydroxyl group.
(2) Boiling Points of Ethers; Hydrogen Bonding
– The most important of The Physical Properties of Ethers is boiling points (no Hydrogen bonds).
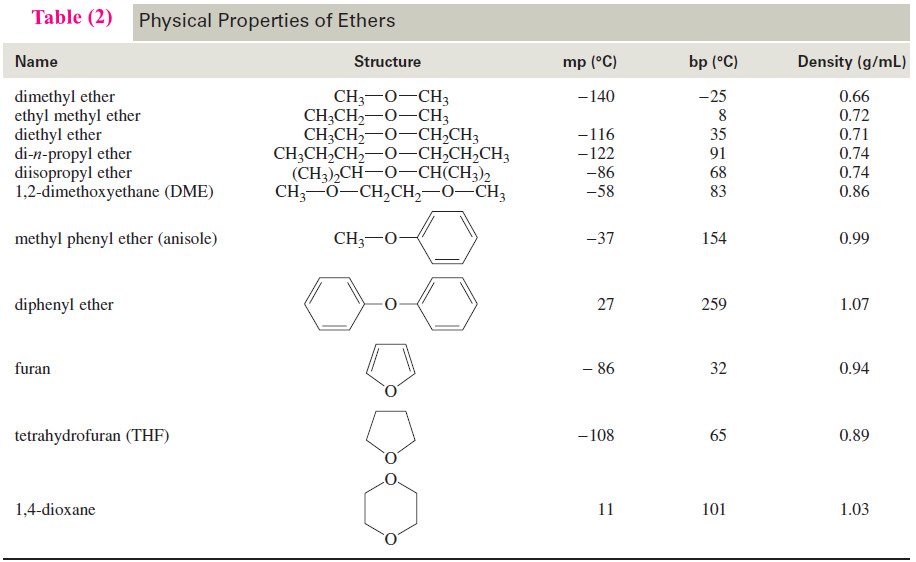
– Table (1) compares the boiling points of several ethers, alcohols, and alkanes.
– Notice that the boiling points of dimethyl ether and diethyl ether are nearly 100 °C lower than those of alcohols having similar molecular weights.
– This large difference results mostly from hydrogen bonding in the alcohols. Pure ethers cannot engage in hydrogen bonding because they have no O-H groups.
– Ethers do have large dipole moments, resulting in dipole–dipole attractions, but these attractions appear to have relatively little effect on their boiling points.
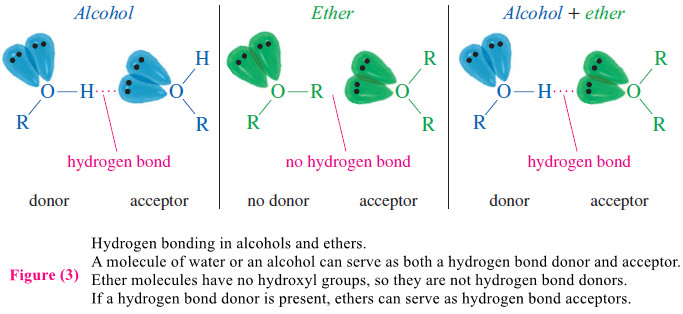
– Although pure ethers have no hydroxyl groups to engage in hydrogen bonding, they can hydrogen bond with other compounds that do have O-H or N-H groups.
– Figure (2) shows that a hydrogen bond requires both a hydrogen bond donor and a hydrogen bond acceptor. The donor is the molecule with an O-H or N-H group.
– The acceptor is the molecule whose lone pair of electrons forms a weak partial bond to the hydrogen atom provided by the donor.
– An ether molecule has the lone pair to form a hydrogen bond with an alcohol (or other hydrogen bond donor), but it cannot form a hydrogen bond with another ether molecule.
– As a result, ethers are much more volatile than alcohols having similar molecular weights.
– Table (2) lists the physical properties of a representative group of common ethers.
(3) Ethers as Polar Solvents
– Ethers are ideally suited as solvents for many organic reactions.
– They dissolve a wide range of polar and nonpolar substances, and their relatively low boiling points simplify their evaporation from the reaction products.
– Nonpolar substances tend to be more soluble in ethers than in alcohols because ethers have no hydrogen-bonding network to be broken up by the nonpolar solute.
– Polar substances tend to be nearly as soluble in ethers as in alcohols because ethers have large dipole moments as well as the ability to serve as hydrogen bond acceptors.
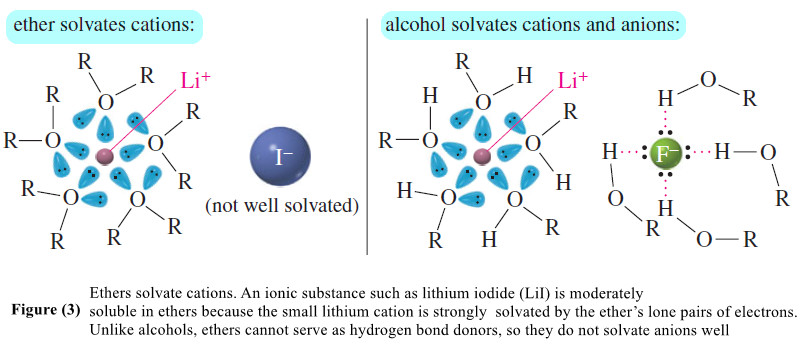
– The nonbonding electron pairs of an ether effectively solvate cations, as shown in Figure (3).
– Ethers do not solvate anions as well as alcohols do, however. Ionic substances with small, “hard” anions requiring strong solvation to overcome their ionic bonding are often insoluble in ether solvents.
– Substances with large, diffuse anions, such as iodides, acetates, and other organic anions, tend to be more soluble in ether solvents than substances with smaller, harder anions such as fluorides.
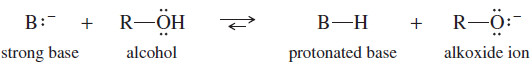
– Alcohols cannot be used as solvents for reagents that are more strongly basic than the alkoxide ion.
– The hydroxyl group quickly protonates the base, destroying the basic reagent.
– Ethers are nonhydroxylic (no hydroxyl group), and they are normally unreactive toward strong bases.
– For this reason, ethers are frequently used as solvents for very strong polar bases (like the Grignard reagent) that require polar solvents.
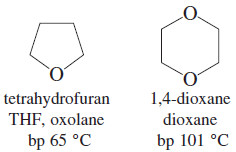
– The four ethers shown here are common solvents for organic reactions.
– DME, THF, and dioxane are miscible with water, and diethyl ether is sparingly soluble in water.
(4) Stable Complexes of Ethers with Reagents
– The special properties of ethers (polarity, lone pairs, but relatively unreactive) enhance the formation and use of many reagents.
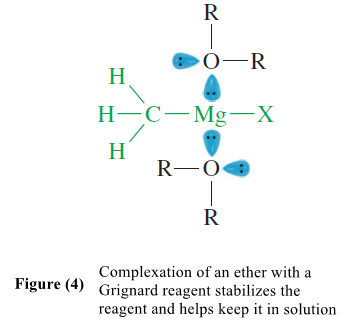
– For example, Grignard reagents cannot form unless an ether is present, possibly to share its lone pairs of electrons with the magnesium atom.
– This sharing of electrons stabilizes the reagent and helps keep it in solution (Figure 4).
Complexes with Electrophiles
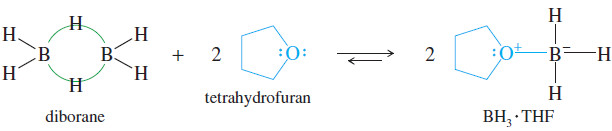
– An ether’s nonbonding electrons also stabilize borane, BH3.
– Pure borane exists as a dimer called diborane, B2H6.
– Diborane is a toxic, flammable, and explosive gas, whose use is both dangerous and inconvenient.
– Borane forms a stable complex with tetrahydrofuran.
– The BH3 . THF complex is commercially available as a 1 M solution, easily measured and transferred like any other air-sensitive liquid reagent.
– The availability of BH3 . THF has contributed greatly to the convenience of hydroboration.
– Boron trifluoride is used as a Lewis acid catalyst in a wide variety of reactions.
– Like diborane, BF3 is a toxic gas, but BF3 forms a stable complex with ethers, allowing it to be conveniently stored and measured.
– The complex of BF3 with diethyl ether is called “boron trifluoride etherate.”
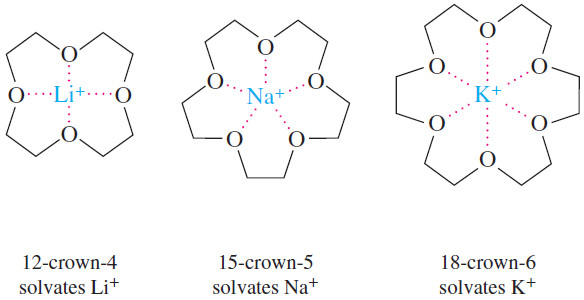
Crown Ether Complexes
– we encountered the use of crown ethers, large cyclic polyethers that specifically solvate metal cations by complexing the metal in the center of the ring.
– Different crown ethers solvate different cations, depending on the relative sizes of the crown ether and the cation and the number of binding sites around the cation.
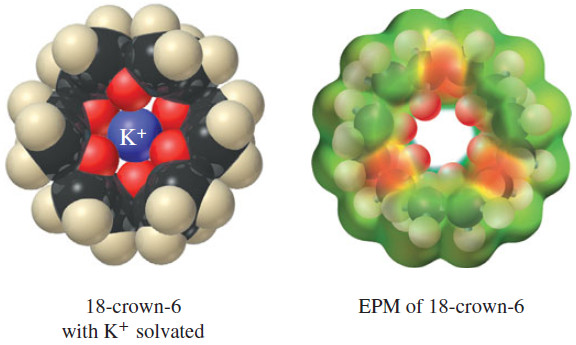
– The EPM of 18-crown-6 shows that the cavity in the center of the molecule is surrounded by electron-rich oxygen atoms that complex with the guest potassium cation.
– Complexation by crown ethers often helps polar inorganic salts to dissolve in nonpolar organic solvents.
– This enhanced solubility allows polar salts to be used under aprotic conditions, where the uncomplexed anions may show greatly enhanced reactivity.
– For example, we used 18-crown-6 to dissolve potassium fluoride in acetonitrile (CH3CN), where the poorly solvated fluoride ion is a moderately strong nucleophile.
– Many other salts, including carboxylate salts (RCOO– +K), cyanides (KCN), and permanganates (KMnO4), can be dissolved in aprotic (and often nonpolar) organic solvents using crown ethers.
– In each case, the crown ether complexes only the cation, leaving the anion bare and highly reactive.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.