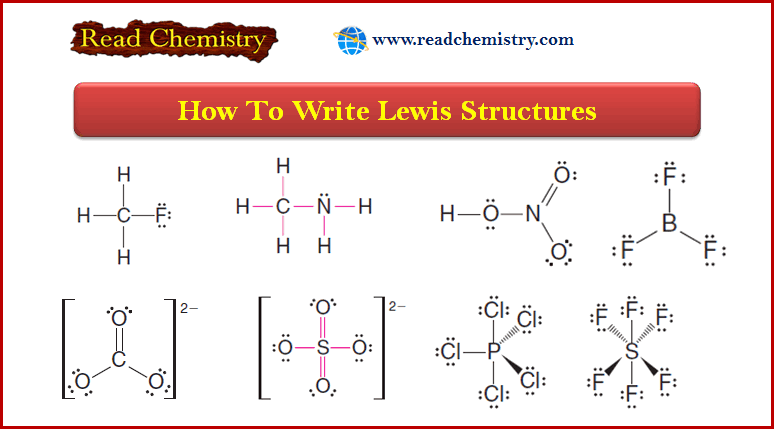
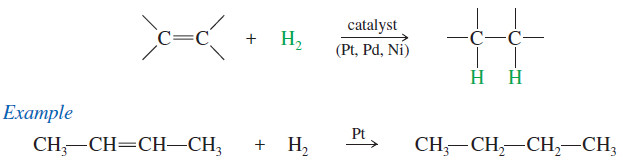Catalytic Hydrogenation of Alkenes
Catalytic Hydrogenation of Alkenes
– Although we have mentioned catalytic hydrogenation before, we now consider the mechanism and stereochemistry in more detail.
– Hydrogenation of an alkene is formally a reduction, with H2 adding across the double bond to give an alkane.
– The process usually requires a catalyst containing Pt, Pd, or Ni.
– For most alkenes, hydrogenation takes place at room temperature, using hydrogen gas at atmospheric pressure.
– The alkene is usually dissolved in an alcohol, an alkane, or acetic acid.
– A small amount of platinum, palladium, or nickel catalyst is added, and the container is shaken or stirred while the reaction proceeds.
– Hydrogenation actually takes place at the surface of the metal, where the liquid solution of the alkene comes into contact with hydrogen and the catalyst.
– Hydrogen gas is adsorbed onto the surface of these metal catalysts, and the catalyst weakens the H-H bond.
– In fact, if H2 and D2 are mixed in the presence of a platinum catalyst, the two isotopes quickly scramble to produce a random mixture of HD, H2 and D2 (No scrambling occurs in the absence of the catalyst.)
– Hydrogenation is an example of heterogeneous catalysis, because the (solid) catalyst is in a different phase from the reactant solution.
– In contrast, homogeneous catalysis involves reactants and catalyst in the same phase, as in the acid-catalyzed dehydration of an alcohol.
– Because the two hydrogen atoms add from a solid surface, they add with syn stereochemistry.
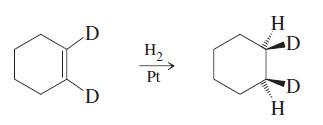
Catalytic hydrogenation of 1,2-dideuteriocyclohexene
– when 1,2-dideuteriocyclohexene is treated with hydrogen gas over a catalyst, the product is the cis isomer resulting from syn addition (Figure below)
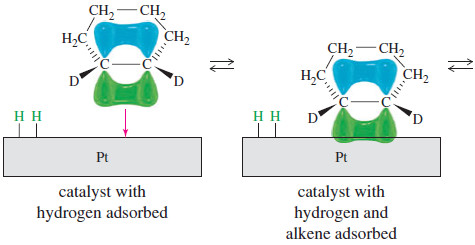
– One face of the alkene pi bond binds to the catalyst, which has hydrogen adsorbed on its surface.
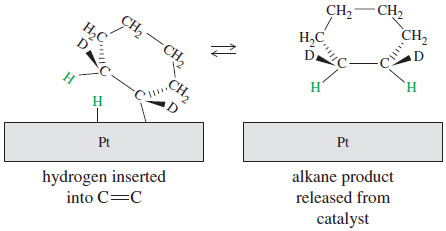
– Hydrogen inserts into the pi bond, and the product is freed from the catalyst.
– Both hydrogen atoms add to the face of the double bond that is complexed with the catalyst.
Stereochemistry in catalytic hydrogenation
– Syn stereochemistry in catalytic hydrogenation.
– A solid heterogeneous catalyst adds two hydrogen atoms to the same face of the pi bond (syn stereochemistry).
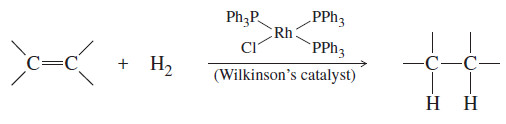
– Soluble homogeneous catalysts, such as Wilkinson’s catalyst, also catalyze the hydrogenation of carbon–carbon double bonds.
– Wilkinson’s catalyst is not chiral, but its triphenylphosphine (PPh3) ligands can be replaced by chiral ligands to give chiral catalysts that are capable of converting optically inactive starting materials to optically active products.
– Such a process is called asymmetric induction or enantioselective synthesis.
– For example, The Figure shows a chiral ruthenium complex catalyzing an enantioselective hydrogenation of a carbon–carbon double bond to give a large excess of one enantiomer.
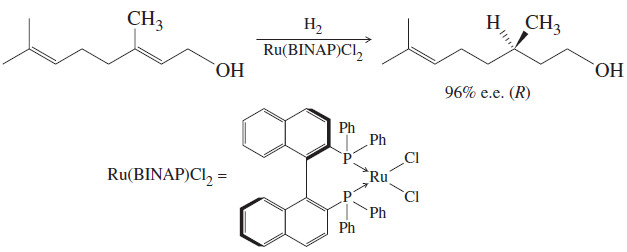
– Because the catalyst is chiral, the transition states leading to the two enantiomers of product are diastereomeric.
– They have different energies, and the transition state leading to the (R) enantiomer is favored.
– Ryoji Noyori and William Knowles shared the 2001 Nobel Prize in Chemistry for their work on chirally catalyzed hydrogenation reactions.
– Enantioselective synthesis is particularly important in the pharmaceutical industry, because only one enantiomer of a chiral drug is likely to have the desired effect.
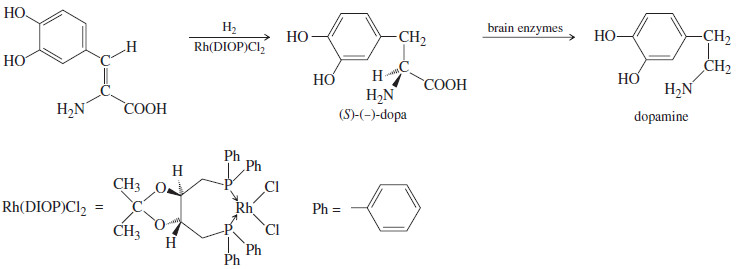
– For example, levodopa [(-)-dopa or l-dopa] is used in patients with Parkinson’s disease to counteract a deficiency of dopamine, one of the neurotransmitters in the brain.
– Dopamine itself is useless as a drug because it cannot cross the “blood brain barrier”; that is, it cannot get into the cerebrospinal fluid from the bloodstream.
(-)-Dopa on the other hand, is an amino acid related to tyrosine.
– It crosses the blood–brain barrier into the cerebrospinal fluid, where it undergoes enzymatic conversion to dopamine.
– Only the (-) enantiomer of dopa can be transformed into dopamine; the other enantiomer, (+)-dopa is toxic to the patient.
– The correct enantiomer can be synthesized from an achiral starting material by catalytic hydrogenation using a complex of rhodium with a chiral ligand called DIOP.
– Such an enantioselective synthesis is more efficient than making a racemic mixture, resolving it into enantiomers, and discarding the unwanted enantiomer.