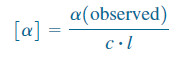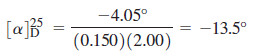Optical Activity in Organic Compounds
– Rotation of the plane of polarized light is called optical activity, and substances that rotate the plane of polarized light are said to be optically active.
– There are alot of Organic Compounds have optical activity
Introduction to Optical activity
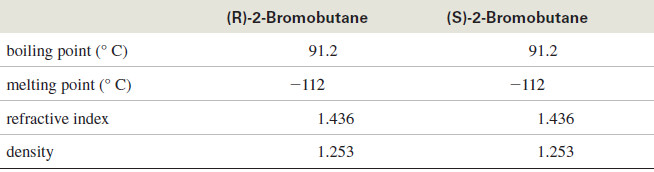
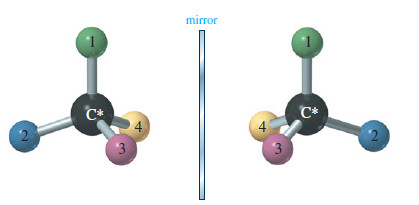
Mirror-image molecules have nearly identical physical properties. Compare the following properties of (R)-2-bromobutane and (S)-2-bromobutane
– Differences in enantiomers become apparent in their interactions with other chiral molecules, such as enzymes.
– Still, we need a simple method to distinguish between enantiomers and measure their purity in the laboratory.
– Polarimetry is a common method used to distinguish between enantiomers, based on their ability to rotate the plane of polarized light in opposite directions.
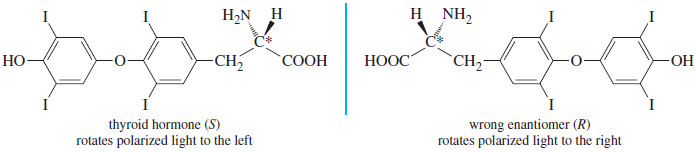
– For example, the two enantiomers of thyroid hormone are shown below.
– The (S) enantiomer has a powerful effect on the metabolic rate of all the cells in the body. The (R) enantiomer is useless.
– In the laboratory, we distinguish between the enantiomers by observing that the active one rotates the plane of polarized light to the left.
Plane-Polarized Light
– Most of what we see is unpolarized light, vibrating randomly in all directions.
– Planepolarized light is composed of waves that vibrate in only one plane.
– Although there are other types of “polarized light,” the term usually refers to plane-polarized light.
– When unpolarized light passes through a polarizing filter, the randomly vibrating light waves are filtered so that most of the light passing through is vibrating in one direction (see Figure ).
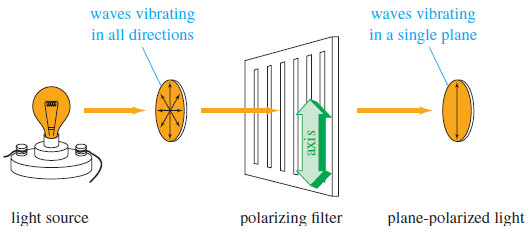
– The direction of vibration is called the axis of the filter.
– Polarizing filters may be made from carefully cut calcite crystals or from specially treated plastic sheets.
– Plastic polarizing filters are often used as lenses in sunglasses, because the axis of the filters can be positioned to filter out reflected glare.
– When light passes first through one polarizing filter and then through another, the amount of light emerging depends on the relationship between the axes of the two filters (see Figure).
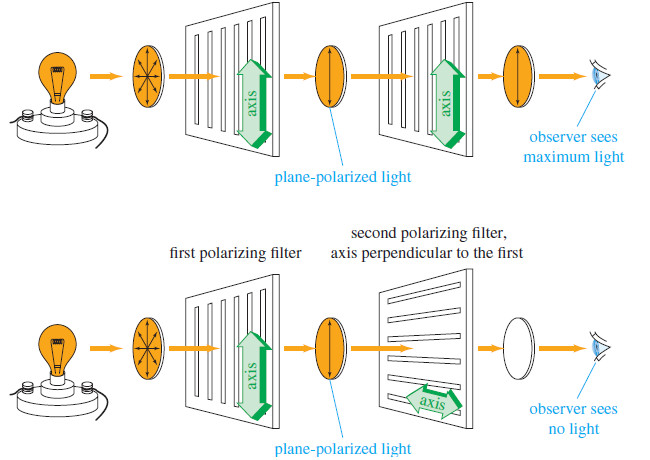
– If the axes of the two filters are lined up (parallel), then nearly all the light that passes through the first filter also passes through the second.
– If the axes of the two filters are perpendicular (crossed poles), however, all the polarized light that emerges from the first filter is stopped by the second.
– At intermediate angles of rotation, intermediate amounts of light pass through.
– You can demonstrate this effect for yourself by wearing a pair of polarized sunglasses while looking at a light source through another pair (see Figure ).
– The second pair seems to be transparent, as long as its axis is lined up with the pair you are wearing.
– When the second pair is rotated to 90°, however, the lenses become opaque, as if they were covered with black ink.
Rotation of Plane-Polarized Light
– When polarized light passes through a solution containing a chiral compound, the chiral compound causes the plane of vibration to rotate.
– Rotation of the plane of polarized light is called optical activity, and substances that rotate the plane of polarized light are said to be optically active.
– Before the relationship between chirality and optical activity was known, enantiomers were called optical isomers because they seemed identical except for their opposite optical activity.
– The term was loosely applied to more than one type of isomerism among optically active compounds, however, and this ambiguous term has been replaced by the well-defined term enantiomers.
– Two enantiomers have identical physical properties, except for the direction they rotate the plane of polarized light.
Enantiomeric compounds rotate the plane of polarized light by exactly the same amount but in opposite directions.
– If the (R) enantiomer rotates the plane 30° clockwise, the (S) enantiomer will rotate it 30° counterclockwise. If the (R) enantiomer rotates the plane 5° counterclockwise, the (S) enantiomer will rotate it 5° clockwise.
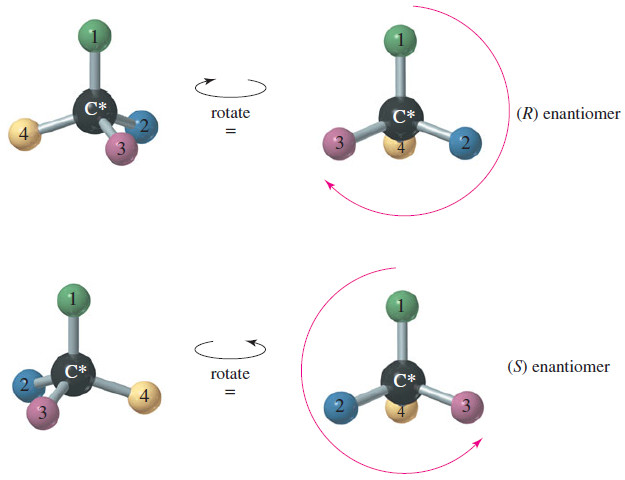
We cannot predict which direction a particular enantiomer [either (R) or (S)] will rotate the plane of polarized light.
– (R) and (S) are simply names, but the direction and magnitude of rotation are physical properties that must be measured.
Polarimetry
– A polarimeter measures the rotation of polarized light.
– It has a tubular cell filled with a solution of the optically active material and a system for passing polarized light through the solution and measuring the rotation as the light emerges (see Figure).
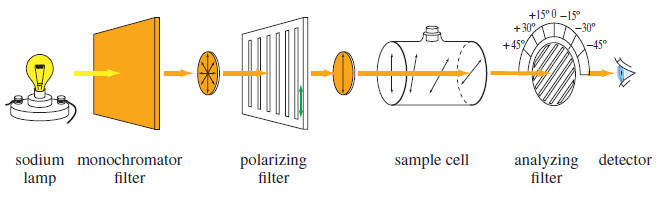
– The light from a sodium lamp is filtered so that it consists of just one wavelength (one color), because most compounds rotate different wavelengths of light by different amounts.
– The wavelength of light most commonly used for polarimetry is a yellow emission line in the spectrum of sodium, called the sodium D line.
– Monochromatic (one-color) light from the source passes through a polarizing filter, then through the sample cell containing a solution of the optically active compound.
– On leaving the sample cell, the polarized light encounters another polarizing filter. This filter is movable, with a scale allowing the operator to read the angle between the axis of the second (analyzing) filter and the axis of the first (polarizing) filter.
– The operator rotates the analyzing filter until the maximum amount of light is transmitted, then reads the observed rotation from the protractor. The observed rotation is symbolized by the Greek letter alpha.
– Compounds that rotate the plane of polarized light toward the right (clockwise) are called dextrorotatory, from the Greek word dexios, meaning “toward the right.”
– Compounds that rotate the plane toward the left (counterclockwise) are called levorotatory, from the Latin word laevus, meaning “toward the left.”
– These terms are sometimes abbreviated by a lowercase d or l.
– Using IUPAC notation, the direction of rotation is specified by the (+) or (-) sign of the rotation:
Dextrorotatory (clockwise) rotations are (+) or (d).
Levorotatory (counterclockwise) rotations are (-) or (l ).
For example, the isomer of butan-2-ol that rotates the plane of polarized light clockwise is named (+)- butan-2-ol or d-butan-2-ol. Its enantiomer, (-)- butan-2-ol or l-butan-2-ol, rotates the plane counterclockwise by exactly the same amount.
You can see the principle of polarimetry by using two pairs of polarized sunglasses, a beaker, and some corn syrup or sugar solution.
– Wear one pair of sunglasses, look down at a light, and hold another pair of sunglasses above the light.
– Notice that the most light is transmitted through the two pairs of sunglasses when their axes are parallel.
– Very little light is transmitted when their axes are perpendicular.
– Put syrup into the beaker, and hold the beaker above the bottom pair of sunglasses so the light passes through one pair of sunglasses (the polarizing filter), then the beaker (the optically active sample), and then the other pair of sunglasses (the analyzing filter); see Figure.
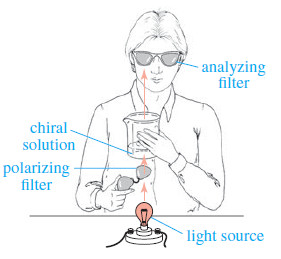
– Again, check the angles giving maximum and minimum light transmission. Is the syrup solution dextrorotatory or levorotatory? Did you notice the color variation as you rotated the filter? You can see why just one color of light should be used for accurate work.
Specific Rotation
– The angular rotation of polarized light by a chiral compound is a characteristic physical property of that compound, just like the boiling point or the density.
– The rotation observed in a polarimeter depends on the concentration of the sample solution and the length of the cell, as well as the optical activity of the compound.
– For example, twice as concentrated a solution would give twice the original rotation. Similarly, a 20-cm cell gives twice the rotation observed using a similar concentration in a 10-cm cell.
To use the rotation of polarized light as a characteristic property of a compound, we must standardize the conditions for measurement.
– We define a compound’s specific rotation [α] as the rotation found using a 10-cm (1-dm) sample cell and a concentration of 1 g mL.
– Other cell lengths and concentrations may be used, as long as the observed rotation is divided by the path length of the cell (l) and the concentration (c).
where:
α (observed) = rotation observed in the polarimeter in grams per mL
c = concentration in grams per mL
l = length of sample cell (path length) in decimeters (dm)
Solved problems on Optical acitivity
When one of the enantiomers of butan-2-ol is placed in a polarimeter, the observed rotation is 4.05° counterclockwise. The solution was made by diluting 6.00 g of butan-2-ol to a total of 40.0 mL, and the solution was placed into a 200-mm polarimeter tube for the measurement. Determine the specific rotation for this enantiomer of butan-2-ol.
Solution
Since it is levorotatory, this must be (-)- butan-2-ol The concentration is 6.00 g per 40.0 mL = 0.150 g/mL, and the path length is 200 mm = 2.00 dm
– The specific rotation is –13.5°.
– A rotation depends on the wavelength of light used and also on the temperature, so these data are given together with the rotation. In Solved Problem , the “25” means that the measurement was made at 25 °C, and the “D” means that the light used was the D line of the sodium spectrum.
– Without even measuring it, we can predict that the specific rotation of the other enantiomer of butan-2-ol will be
where the (+) refers to the clockwise direction of the rotation. This enantiomer would be called (+)- butan-2-ol
– We could refer to this pair of enantiomers as (+)- butan-2-ol and (-)- butan-2-ol or as (R)-butan-2-ol and (S)-butan-2-ol.
– Does this mean that (R)-butan-2-ol is the dextrorotatory isomer because it is named (R), and (S)-butan-2-ol is levorotatory because it is named (S)? Not at all!
– The rotation of a compound, (+) or (-) is something that we measure in the polarimeter, depending on how the molecule interacts with light.
– The (R) and (S) nomenclature is our own artificial way of describing how the atoms are arranged in space.
– In the laboratory, we can measure a rotation and see whether a particular substance is (+) or (-)
– On paper, we can determine whether a particular drawing is named (R) or (S).
– But it is difficult to predict whether a structure we call (R) will rotate polarized light clockwise or counterclockwise.
– Similarly, it is difficult to predict whether a dextrorotatory substance in a flask has the (R) or (S) configuration.