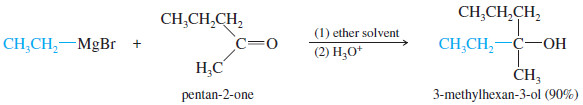Addition of Grignard Reagents to Carbonyl Compounds
Addition of Organometallic Reagents to Carbonyl Compounds
– Because they resemble carbanions, Grignard reagents and organolithium reagents are strong nucleophiles and strong bases.
– Their most useful nucleophilic reactions are additions to carbonyl (C=O) groups, much like we saw with acetylide ions
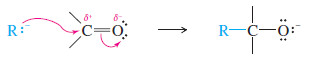
– The carbonyl group is polarized, with a partial positive charge on carbon and a partial negative charge on oxygen.
– The positively charged carbon is electrophilic; attack by a nucleophile places a negative charge on the electronegative oxygen atom.
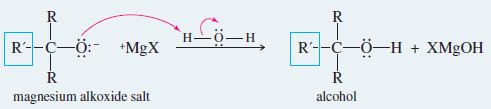
– The product of this nucleophilic attack is an alkoxide ion, a strong base.
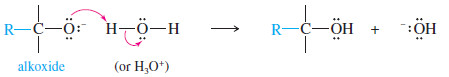
– Addition of water or a dilute acid in a second step protonates the alkoxide to give the alcohol.
– Either a Grignard reagent or an organolithium reagent can serve as the nucleophile in this addition to a carbonyl group.
– The following discussions refer to Grignard reagents, but they also apply to organolithium reagents.
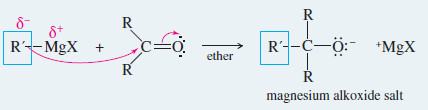
– Key Mechanism shows that the Grignard reagent first adds to the carbonyl group to form an alkoxide ion.
– Addition of dilute acid (in a separate step) protonates the alkoxide to give the alcohol.
– We are interested primarily in the reactions of Grignard reagents with ketones and aldehydes.
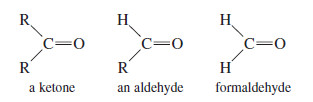
– Ketones are compounds with two alkyl groups bonded to a carbonyl group.
– Aldehydes have one alkyl group and one hydrogen atom bonded to the carbonyl group.
– Formaldehyde has two hydrogen atoms bonded to the carbonyl group
The electrostatic potential map (EPM) of formaldehyde shows the polarization of the carbonyl group, with an electron-rich (red) region around oxygen and an electron-poor (blue) region near carbon.
Mechanism of Grignard Reagents Reactions
– Grignard and organolithium reagents provide some of the best methods for assembling a carbon skeleton.
– These strong nucleophiles add to ketones and aldehydes to give alkoxide ions, which are protonated to give alcohols.
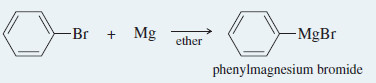
– Formation of the Grignard reagent: Magnesium reacts with an alkyl halide in an anhydrous ether solution.
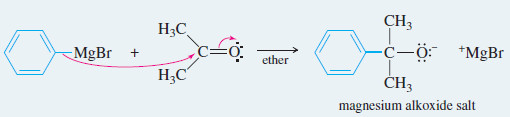
Reaction 1: The Grignard reagent attacks a carbonyl compound to form an alkoxide salt.
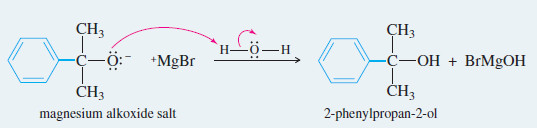
Reaction 2: After the first reaction is complete, water or dilute acid is added to protonate the alkoxide and give the alcohol
Example: Addition of phenylmagnesium bromide to acetone.
Formation of the Grignard reagent: Magnesium reacts with bromobenzene in an ether solution to give phenylmagnesium bromide
Reaction 1: The Grignard reagent attacks a carbonyl compound to form an alkoxide salt
Reaction 2: After the first reaction is complete, water or dilute acid is added to protonate the alkoxide and give the alcohol
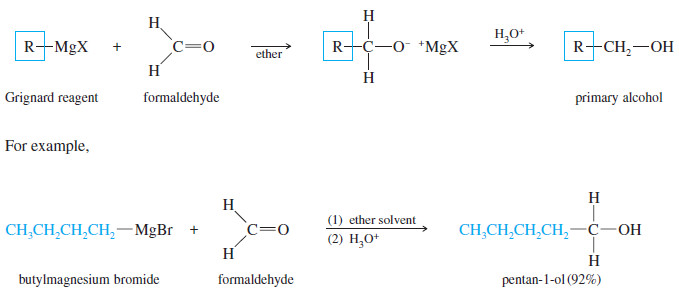
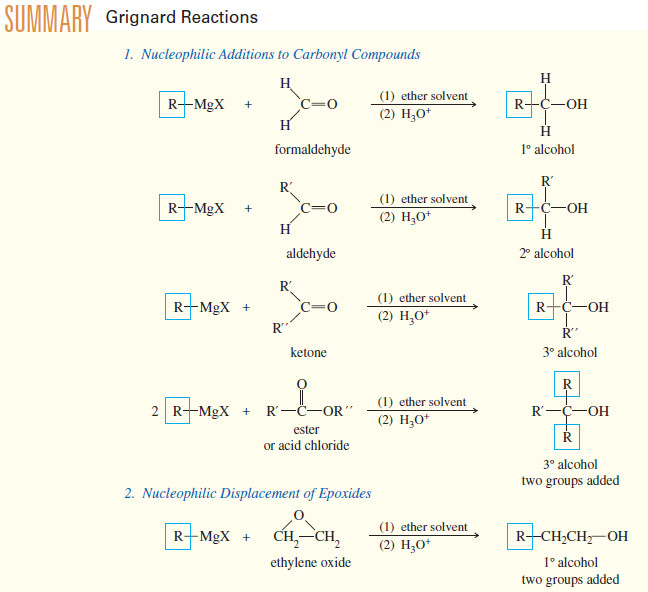
(A) Addition to Formaldehyde: Formation of Primary Alcohols
– Addition of a Grignard reagent to formaldehyde, followed by protonation, gives a primary alcohol with one more carbon atom than in the Grignard reagent
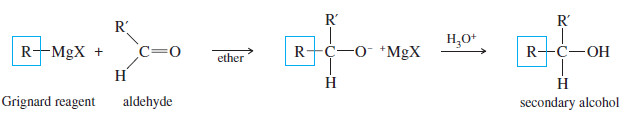
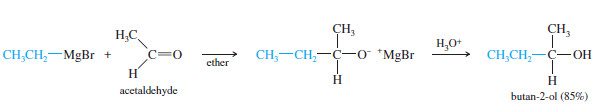
(B) Addition to Aldehydes: Formation of Secondary Alcohols
– Grignard reagents add to aldehydes to give, after protonation, secondary alcohols.
– The two alkyl groups of the secondary alcohol are the alkyl group from the Grignard reagent and the alkyl group that was bonded to the carbonyl group of the aldehyde.
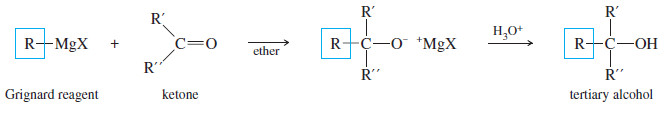
(C) Addition to Ketones: Formation of Tertiary Alcohols
– A ketone has two alkyl groups bonded to its carbonyl carbon atom.
– Addition of a Grignard reagent, followed by protonation, gives a tertiary alcohol, with three alkyl groups bonded to the carbinol carbon atom.
– Two of the alkyl groups are the two originally bonded to the ketone carbonyl group.
– The third alkyl group comes from the Grignard reagent.
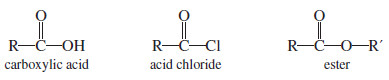
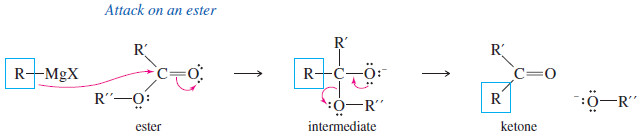
(D) Addition to Acid Chlorides and Esters
– Acid chlorides and esters are derivatives of carboxylic acids.
– In such acid derivatives, the -OH group of a carboxylic acid is replaced by other electron-withdrawing groups.
– In acid chlorides, the hydroxyl group of the acid is replaced by a chlorine atom.
– And In esters, the hydroxyl group is replaced by an alkoxyl (-O-R) group.
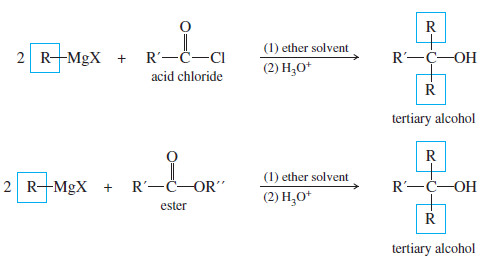
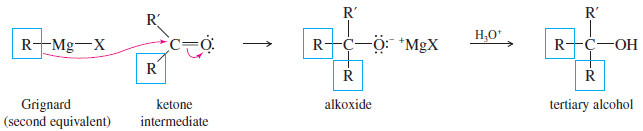
– Acid chlorides and esters react with two equivalents of Grignard reagents to give (after protonation) tertiary alcohols.
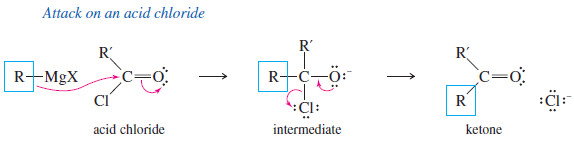
– Addition of the first equivalent of the Grignard reagent produces an unstable intermediate that expels a chloride ion (in the acid chloride) or an alkoxide ion (in the ester), to give a ketone.
– The alkoxide ion is a suitable leaving group in this reaction because its leaving stabilizes a negatively charged intermediate in a fast, strongly exothermic step.
– The ketone reacts with a second equivalent of the Grignard reagent, forming the magnesium salt of a tertiary alkoxide.
– Protonation gives a tertiary alcohol with one of its alkyl groups derived from the acid chloride or ester, and the other two derived from the Grignard reagent
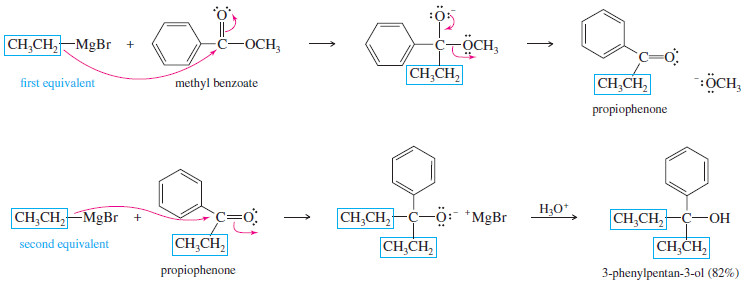
– Consider an example using an ester.
– When an excess of ethylmagnesium bromide is added to methyl benzoate, the first equivalent adds and methoxide is expelled, giving propiophenone.
– Addition of a second equivalent, followed by protonation, gives a tertiary alcohol: 3-phenylpentan-3-ol.
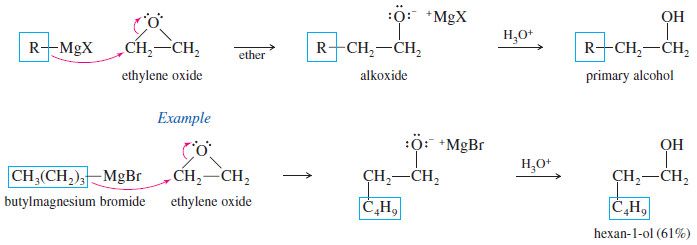
(E) Addition Grignard Reagents to Ethylene Oxide
– Grignard reagents usually do not react with ethers, but epoxides are unusually reactive ethers because of their ring strain.
– Ethylene oxide reacts with Grignard reagents to give, after protonation, primary alcohols with two additional carbon atoms.
– Notice that the nucleophilic attack by the Grignard reagent opens the ring and relieves the ring strain.