Oxidation of Alcohols
– Primary and secondary alcohols are easily oxidized (Oxidation of Alcohols) by a variety of reagents, including chromium oxides, permanganate, nitric acid, and even household bleach (NaOCl, sodium hypochlorite).
– The choice of reagent depends on the amount and value of the alcohol.
– We use cheap oxidants for large-scale oxidations of simple, inexpensive alcohols.
– We use the most effective and selective reagents, regardless of cost, for delicate and valuable alcohols.
– Many of the traditional oxidants are based on chromium(VI) compounds.
– These chromium reagents are highly toxic, and they are difficult to dispose of properly.
– Chemists are gradually moving to less toxic oxidants.
– We will cover the traditional chromium reagents and their uses, and then we will survey the more environmentally friendly alternatives.
– Although this may seem like a bewildering array of oxidizing agents, all of them have an element (Cr, Cl, I, or S) in a high oxidation state bonded to oxygen.
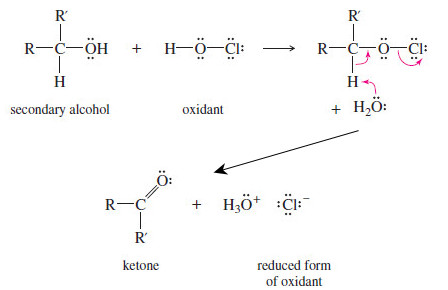
– Moreover, they follow similar mechanisms, as illustrated by the reaction of hypochlorous acid (HOCl, from household bleach), with a secondary alcohol:
– The first step forms an intermediate in which the alcohol oxygen replaces one of the oxidant’s original bonds to oxygen.
– In the next step, a base (often water or other solvent) removes a proton from the carbinol carbon atom, giving it a double bond to oxygen, which leaves it oxidized.
– The oxidant leaves with fewer bonds to oxygen and one more pair of electrons, giving it a lower (reduced) oxidation state.
(A) Oxidation of Secondary Alcohols
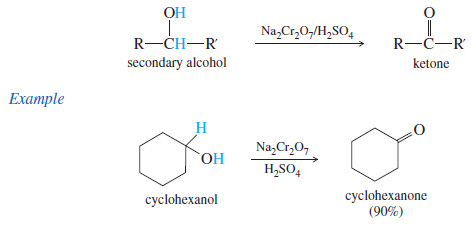
– Secondary alcohols are easily oxidized to give excellent yields of ketones.
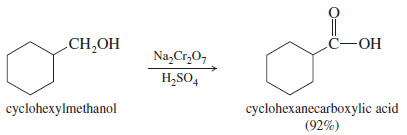
– The chromic acid reagent is often used for laboratory oxidations of secondary alcohols
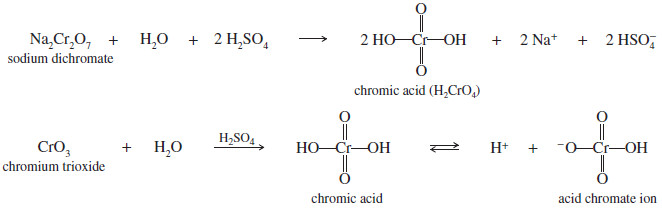
– The chromic acid reagent is prepared by dissolving sodium dichromate (Na2Cr2O7) in a mixture of sulfuric acid and water.
– The active species in the mixture is probably chromic acid H2CrO4 , or the acid chromate ion, H2CrO4– .
– Adding chromium trioxide CrO3 to dilute sulfuric acid achieves the same result
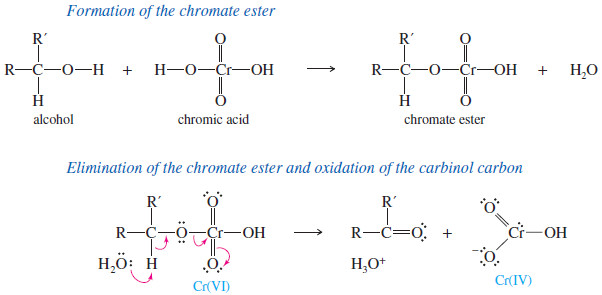
– The mechanism of chromic acid oxidation probably involves the formation of a chromate ester.
– Elimination of the chromate ester gives the ketone.
– In the elimination, the carbinol carbon retains its oxygen atom but loses its hydrogen and gains the second bond to oxygen
– The chromium(IV) species formed reacts further to give the stable reduced form, chromium(III).
– Both sodium dichromate and chromic acid are orange, but chromic ion (Cr3+) is a deep blue.
– One can follow the progress of a chromic acid oxidation by observing the color change from orange through various shades of green to a greenish blue.
– In fact, the color change observed with chromic acid can be used as a test for the presence of an oxidizable alcohol
(B) Oxidation of Primary Alcohols
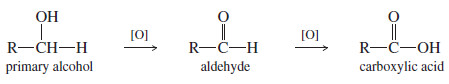
– Oxidation of a primary alcohol initially forms an aldehyde.
– Unlike a ketone, however, an aldehyde is easily oxidized further to give a carboxylic acid.
– Obtaining the aldehyde is often difficult, since most oxidizing agents strong enough to oxidize primary alcohols also oxidize aldehydes.
– Chromic acid generally oxidizes a primary alcohol all the way to the carboxylic acid
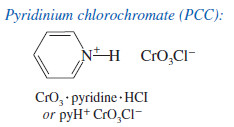
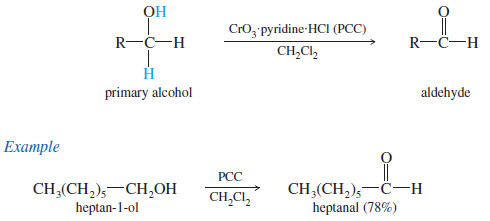
– A better reagent for the limited oxidation of primary alcohols to aldehydes is pyridinium chlorochromate (PCC), a complex of chromium trioxide with pyridine and HCl.
– PCC oxidizes most primary alcohols to aldehydes in excellent yields.
– Unlike most other oxidants, PCC is soluble in nonpolar solvents such as dichloromethane (CH2Cl2) which is an excellent solvent for most organic compounds.
– PCC can also serve as a mild reagent for oxidizing secondary alcohols to ketones
(C) Resistance of Tertiary Alcohols to Oxidation
– Oxidation of tertiary alcohols is not an important reaction in organic chemistry.
– Tertiary alcohols have no hydrogen atoms on the carbinol carbon atom, so oxidation must take place by breaking carbon–carbon bonds.
– These oxidations require severe conditions and result in mixtures of products.
– The chromic acid test for primary and secondary alcohols exploits the resistance of tertiary alcohols to oxidation.
– When a primary or secondary alcohol is added to the chromic acid reagent, the orange color changes to green or blue.
– When a nonoxidizable substance (such as a tertiary alcohol, a ketone, or an alkane) is added to the reagent, no immediate color change occurs
 Read Chemistry
Read Chemistry