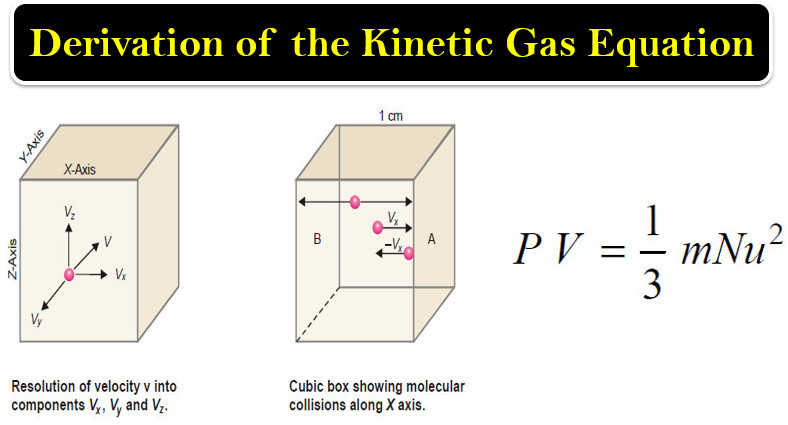
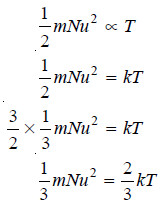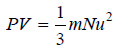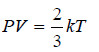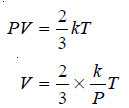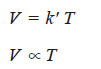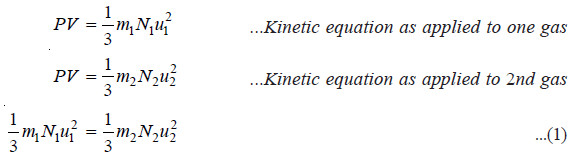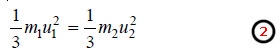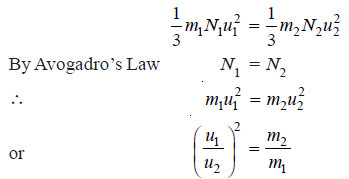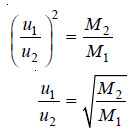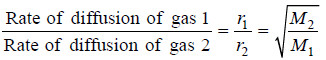Deduction of Gas laws from the Kinetic Gas Equation
– In this topic, we will talk about Deduction of Gas laws from the Kinetic Gas Equation.
Assumptions of the Kinetic Molecular Theory
(1) A gas consists of extremely small discrete particles called molecules dispersed throughout the container.
– The actual volume of the molecules is negligible compared to the total volume of the gas. The molecules of a given gas are identical and have the same mass (m).
(2) Gas molecules are in constant random motion with high velocities.
– They move in straight lines with uniform velocity and change direction on collision with other molecules or the walls of the container.
(3) The distance between the molecules are very large and it is assumed that van der Waals attractive forces between them do not exist. Thus the gas molecules can move freely, independent of each other.
(4) All collisions are perfectly elastic. Hence, there is no loss of the kinetic energy of a molecule during a collision.
(5) The pressure of a gas is caused by the hits recorded by molecules on the walls of the container.
(6) The average kinetic energy (1/2 mv2) of the gas molecules is directly proportional to absolute temperature (Kelvin temperature). This implies that the average kinetic energy of molecules is the same at a given temperature
Deduction of Gas laws from the Kinetic Gas Equation
(1) Boyle’s Law
– According to the Kinetic Theory, there is a direct proportionality between absolute temperature and average kinetic energy of the molecules i.e.,
– Substituting the above value in the kinetic gas equation:
– we have:
– The product PV, therefore, will have a constant value at a constant temperature. This is Boyle’s Law.
(2) Charles’ Law
– As derived above,
– At constant pressure,
– That is, at constant pressure, volume of a gas is proportional to Kelvin temperature and this is Charles’ Law.
(3) Avogadro’s Law
– If equal volume of two gases be considered at the same pressure,
– When the temperature (T) of both the gases is the same, their mean kinetic energy per molecule will also be the same.
– Dividing (1) by (2), we have
N1 = N2
– Or, under the same conditions of temperature and pressure, equal volumes of the two gases contain the same number of molecules. This is Avogadro’s Law.
(4) Graham’s Law of Diffusion
– If m1 and m2 are the masses and u1 and u2 the velocities of the molecules of gases 1 and 2, then at the same pressure and volume:
– If M1 and M2 represent the molecular masses of gases 1 and 2,
– The rate of diffusion (r) is proportional to the velocity of molecules (u), Therefore,
– This is Graham’s Law of Diffusion.
References
- Atkins’ Physical Chemistry / Peter Atkin, Julio de Paula, James Keeler / 12th edition, 2022 / Oxford University Press, UK.
- Physical Chemistry/ Robert G. Mortimer/ 3rd Edition / 2008/ Elsevier Inc, USA.
- Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition/ S. Chand Publishing co / india.
- Physical chemistry for the chemical sciences / Raymond Chang, John W. Thoman, Jr./1st edition, 2014/ University Science Books, USA