Isotopes: Definition, representation, Examples
– In this subject, we will discuss the Isotopes: Definition, representation, Examples
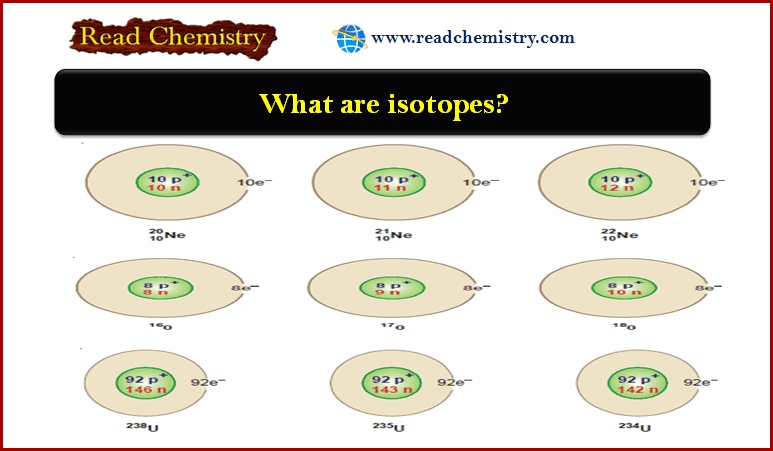
What are Isotopes?
– Contrary to Dalton’s Atomic theory, all atoms of a given element are not necessarily identical.
– Most elements are composed of two or more types of atoms mixed in a fixed proportion.
– The different atoms of such an element contain an equal number of protons and, therefore, have the same atomic number.
– The atoms which vary from one another have different numbers of neutrons in the nucleus.
– Thus they have different atomic masses.
– isotopes may be defined as:
(1) The atoms of an element that have the same number of protons and different numbers of neutrons are called Isotopes.
(2) The atoms of an element that have the same atomic number but different atomic masses or mass numbers.
– The name ‘isotope’ was assigned to them by Soddy because they have the same atomic number and hence occupy the same place in the periodic table (Greek, isos = same; topos = place).
– Isotopes have similar chemical properties as they have the same electronic configuration.
– However, they differ in physical properties which depend on atomic mass.
Symbolic representation of isotopes
– In denoting particular isotopes of an element, the following notation has been internationally adopted.
– The symbol of the element is written with atomic mass at the head and atomic number at the bottom.
– Alternatively, the name of the element is followed by the atomic mass with a hyphen (-) in between.
– EX: Thus the isotopes of carbon (atomic number 6) having atomic masses 12 and 14 may be written as:
– 12C or Carbon-12 reads ‘carbon twelve’, meaning isotope of carbon with a mass of approximately 12 amu.
Examples of isotopes
– Since isotopes of an element have the same atomic number, each of these contains an equal number of protons.
– They have different atomic masses which is accounted for by the different number of neutrons present in the nucleus.
– Thus the isotopes of an element are characterized by different numbers of neutrons in the nucleus.
– The atomic structure of an isotope with atomic number Z and mass number A (atomic mass in amu) can be given as follows:
(1) The number of extranuclear electrons = Z
(2) The number of protons in the nucleus = Z
(3) The mass number A is equal to the total number of protons (Z) and neutrons (N) in the nucleus. That is,
A = Z + N
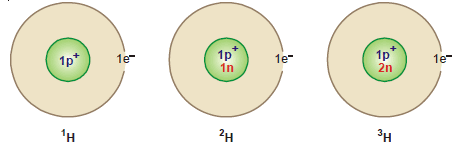
Hydrogen
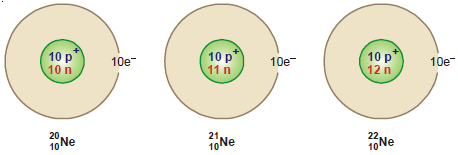
Neon
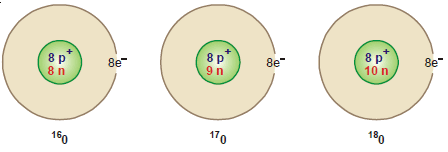
Oxygen
Chlorine
Uranium
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.