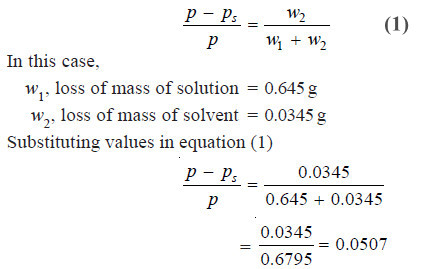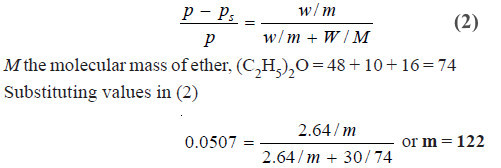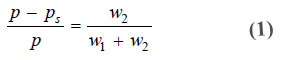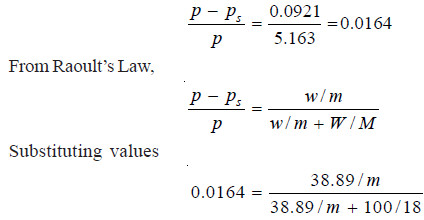Measurement of lowering of vapour pressure
MEASUREMENT OF LOWERING OF VAPOUR PRESSURE
(1) Barometric Method
– Raoult measured the individual vapour pressure of a liquid and then the solution by this method.
– He introduced the liquid or the solution into Toricellian vacuum of a barometer tube and measured the depression of the mercury level.
– This method is neither practicable nor accurate as the lowering of vapour pressure is too small.
(2) Manometric Method
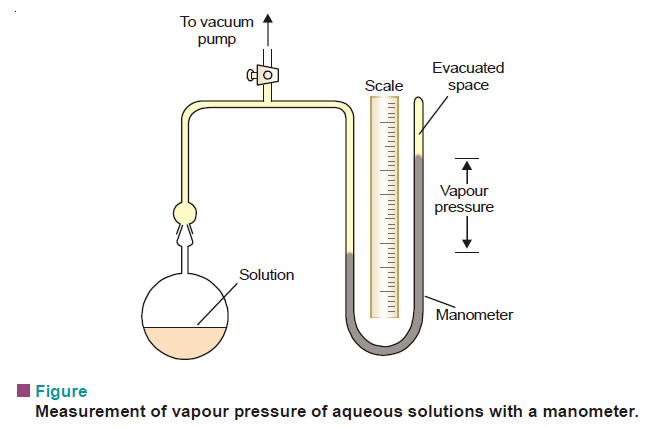
– The vapour pressure of a liquid or solution can be conveniently measured with the help of a manometer (see Fig).
– The bulb B is charged with the liquid or solution. The air in the connecting tube in then removed with a vacuum pump.
– When the stopcock is closed, the pressure inside is due only to the vapour evaporating from the solution or liquid.
– This method is generally used for aqueous solutions.
– The manometric liquid can be mercury or n-butyl phthalate which has low density and low volatility.
(3) Ostwald and Walker’s Dynamic Method (Gas Saturation Method)
– In this method the relative lowering of vapour pressure can be determined straightway.
– The measurement of the individual vapour pressures of a solution and solvent is thus eliminated.
Procedure
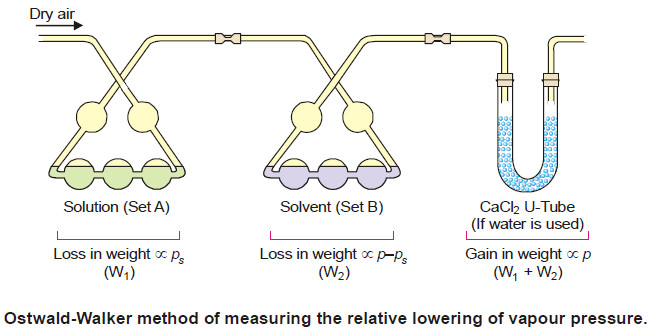
The apparatus used by Ostwald and Walker is shown in the following Fig.
– It consists of two sets of bulbs :
(a) Set (A) containing the solution
(b) Set (B) containing the solvent
– Each set is weighed separately.
– A slow stream of dry air is then drawn by suction pump through the two sets of bulbs.
– At the end of the operation, these sets are reweighed. From the loss of weight in each of the two sets, the lowering of vapour pressure is calculated.
– The temperature of the air, the solution and the solvent must be kept constant throughout.
Calculations
– As the air bubbles through set (A) it is saturated up to the vapour pressure ps of solution and then up to vapour pressure p of solvent in set (B).
– Thus the amount of solvent taken up in set (A) is proportional to ps and the amount taken up in set (B) is proportional to (p – ps).
– If w1 and w2 be the loss of weight in set A and B respectively,
w1 ∝ ps …(1)
w2 ∝ p – ps …(2)
Adding (1) and (2),we have
w1 + w2 ∝ ps + p – ps
∝ p …(3)
Dividing (2) by (3), we can write
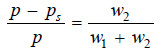
– Knowing the loss of mass in set B (w2) and the total loss of mass in the two sets (w1 + w2), we can find the relative lowering of vapour pressure from equation (4).
– If water is the solvent used, a set of calcium chloride tubes (or a set of bulbs containing conc. H2SO4) is attached to the end of the apparatus to catch the escaping water vapour. Thus the gain in mass of the CaCl2-tubes is equal to (w1 + w2), the total loss of mass in sets (A) and (B).
SOLVED PROBLEM
Problem (1): A current of dry air was passed through a solution of 2.64 g of benzoic acid in 30.0 g of ether (C2H5OC2H5) and then through pure ether. The loss in weight of the solution was 0.645 g and the ether 0.0345 g. What is the molecular mass of benzoic acid ?
solution
According to the theory of Ostwald-Walker method,
From Raoult’s Law, we have
Hence, m, the molecular mass of benzoic acid = 122
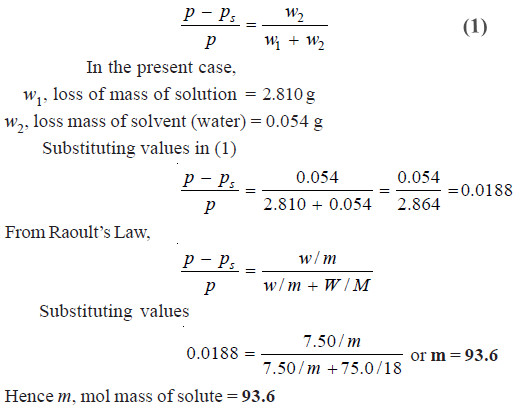
Problem (2): A stream of dry air was passed through a bulb containing a solution of 7.50 g of an aromatic compound in 75.0 g of water and through another globe containing pure water. The loss in mass in the first globe was 2.810 g and in the second globe it was 0.054 g. Calculate the molecular mass of the aromatic compound. (Mol mass of water = 18)
solution
According to the theory of Ostwald-Walker method,
Problem (3): In an experiment air was drawn successively through a solution of sugar (38.89 g per 100 g water) and distilled water, and then through anhydrous calcium chloride. It was found that the water lost was 0.0921 g and calcium chloride tubes gained 5.163 g. Find the molecular mass of the sugar. (Mol mass of H2O = 18)
solution
According to the theory of Ostwald-Walker method,
In this case,
w2, the loss of mass of water = 0.0921 g
(w1 + w2), the total loss of solution and solvent = 5.163 g
Substituting values in equation (1)
Hence m, mol mass of sugar = 385