-
Physical Chemistry
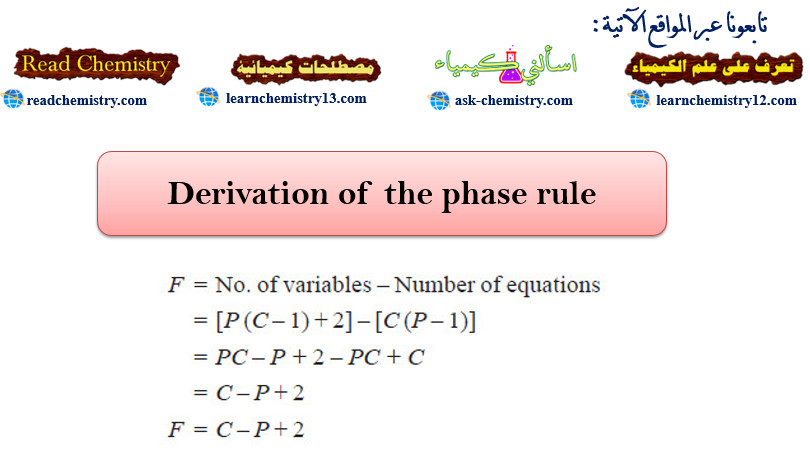
Derivation of the phase rule
Derivation of the phase rule – Here the derivation of the phase rule for one-component system and two-component system are…
Read More » -
Physical Chemistry
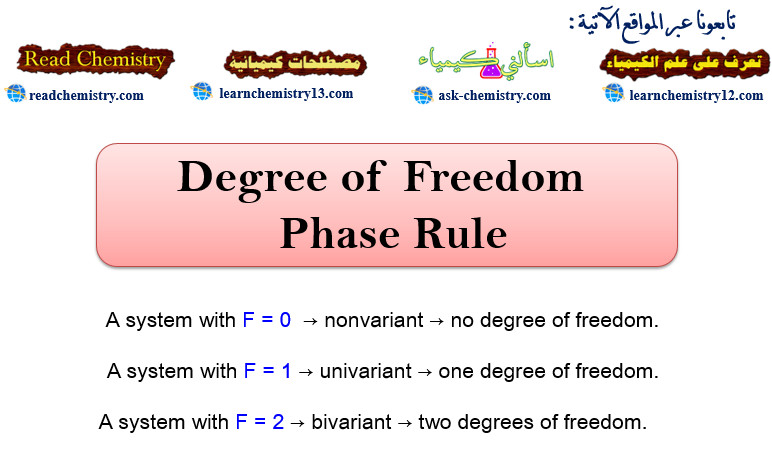
Degree of Freedom – phase Rule
Degree of Freedom – The term Degree of Freedom represented by F in the phase Rule equation (F = C…
Read More » -
Physical Chemistry
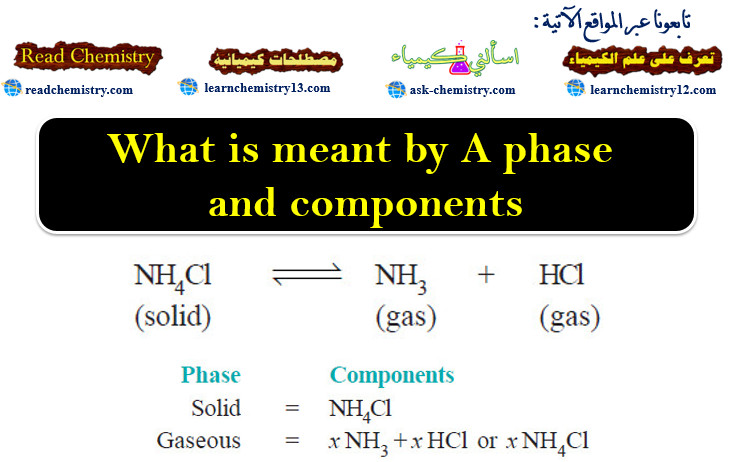
What is meant by A phase and components?
The Phase Rule statement – The phase Rule is an important generalization dealing with the behaviour of heterogeneous systems. –…
Read More » -
Organic Chemistry
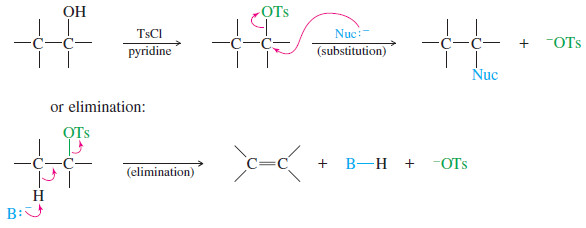
Alcohols as Nucleophiles and Electrophiles
Alcohols as Nucleophiles and Electrophiles; Formation of Tosylates – One reason alcohols are such versatile chemical intermediates is that they…
Read More » -
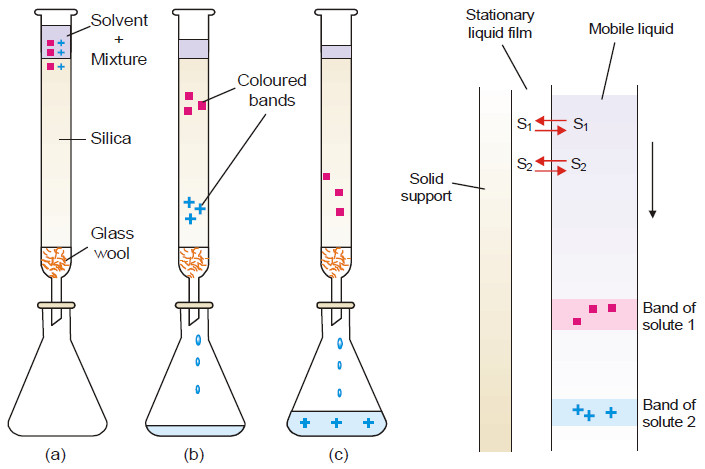
Physical Chemistry
Applications of distribution law
Applications of distribution law – There are numerous applications of distribution law in the laboratory as well as in industry.…
Read More » -
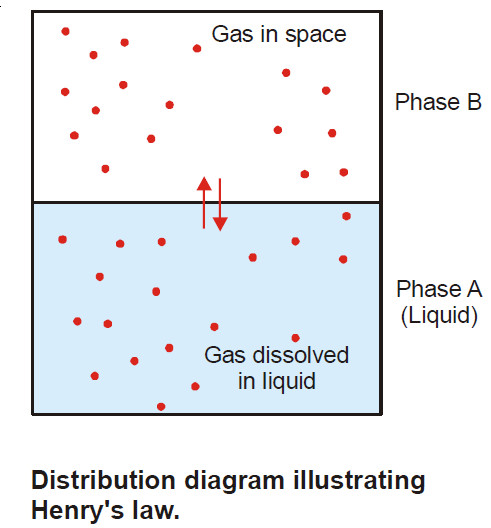
Physical Chemistry
Henry’s law – a form of distribution law
Henry’s law statement – Henry’s law states: at a constant temperature the solubility of a gas in a liquid is…
Read More » -
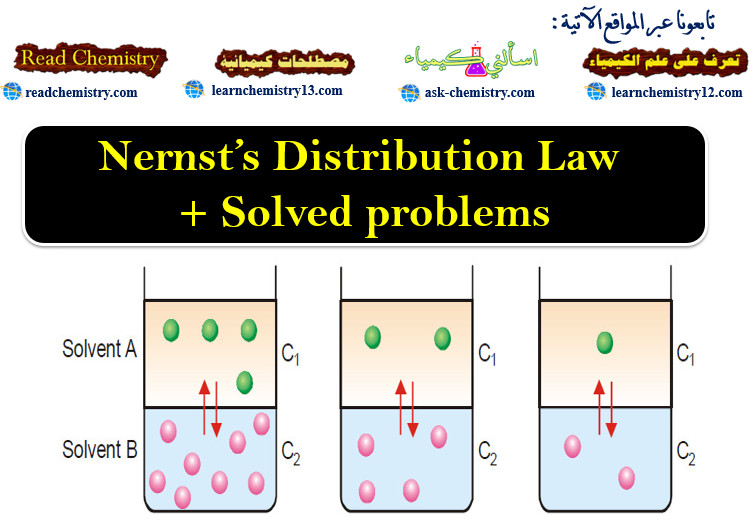
Physical Chemistry
Nernst’s Distribution Law + Solved problems
Introduction to Nernst’s Distribution Law – In this topic Nernst’s Distribution Law wlll be discussed – If we take two…
Read More » -
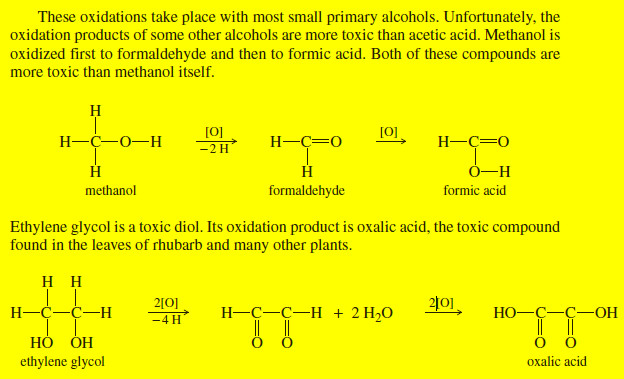
Organic Chemistry
Biological Oxidation of Alcohols
– In this topic, the Biological Oxidation of Alcohols and their effect on the humans and animals will be discussed…
Read More » -
Physical Chemistry
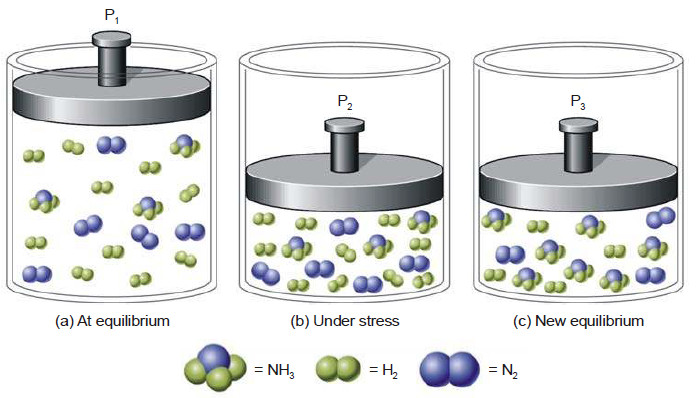
Le Chatelier’s principle
Le Chatelier’s principle – In 1884, the French Chemist Henry Le Chatelier proposed a general principle which applies to all…
Read More » -
Organic Chemistry
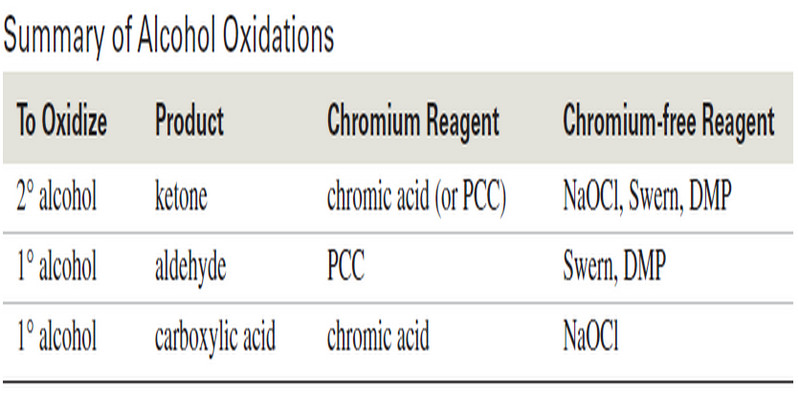
Additional Methods for Oxidizing Alcohols
Additional Methods for Oxidizing Alcohols – Many other reagents and procedures have been developed for oxidizing alcohols. – Some are…
Read More » -
Physical Chemistry
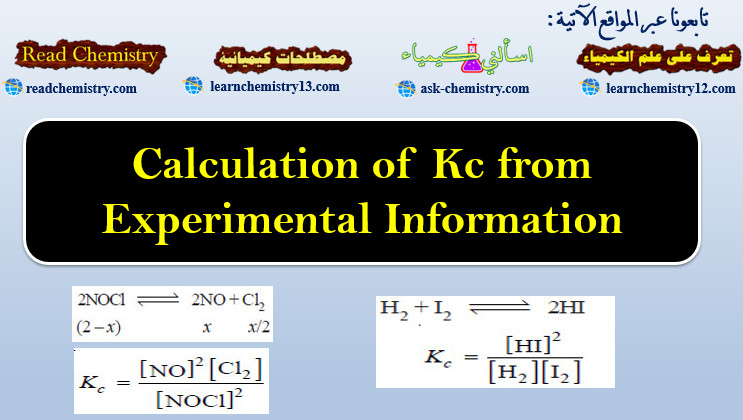
Calculation of Kc from Experimental Information
Calculation of Kc from Experimental Information – To determine the value of Kc of a reaction, write the balanced equation.…
Read More » -
Organic Chemistry
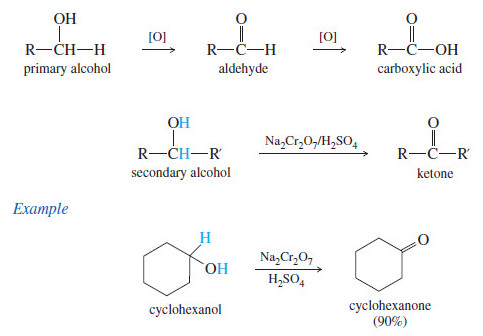
Oxidation of Alcohols
Oxidation of Alcohols – Primary and secondary alcohols are easily oxidized (Oxidation of Alcohols) by a variety of reagents, including…
Read More » -
Physical Chemistry
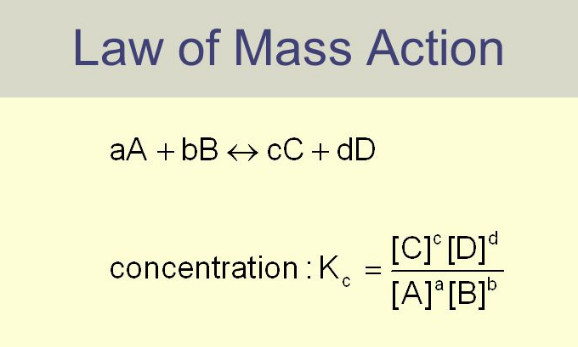
Equilibrium Constant Expression
Equilibrium constant: Equilibrium law – Now we will find the Expression of Equilibrium Constant. – Let us consider a general…
Read More » -
Organic Chemistry
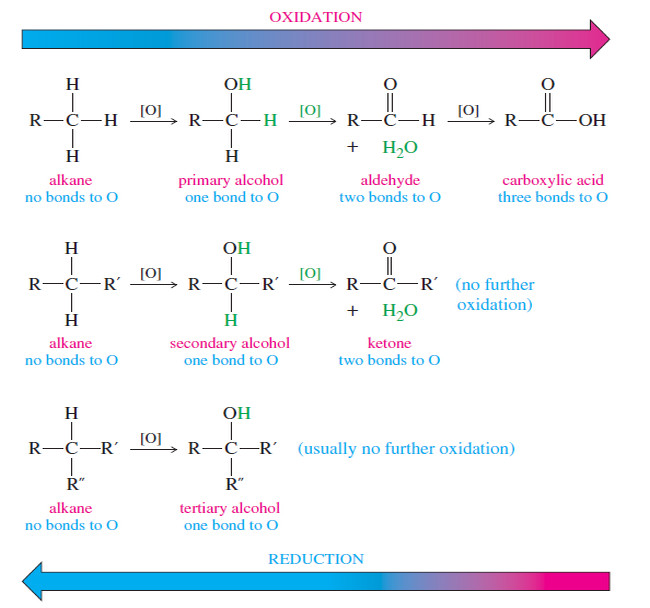
Oxidation states of Alcohols and Related Functional Groups
Oxidation states of Alcohols and Related Functional Groups – Oxidation states of Alcohols leads to ketones, aldehydes, and carboxylic acids.…
Read More » -
Organic Chemistry
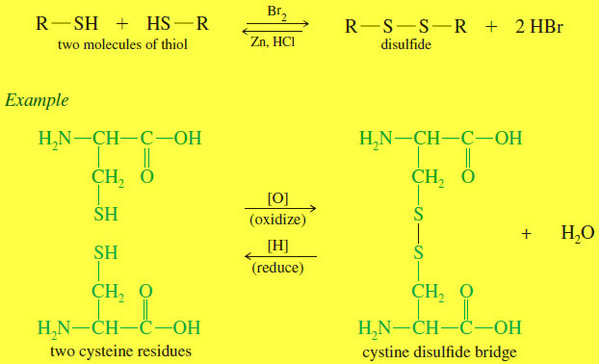
Thiols (Mercaptans)
What is Thiols? – Thiols are sulfur analogues of alcohols, with an -SH group in place of the alcohol -OH…
Read More » -
Physical Chemistry
Law of Mass action
Law of Mass action – Two Norwegian chemists, Guldberg and Waage, studied experimentally a large number of equilibrium reactions. In…
Read More » -
Organic Chemistry
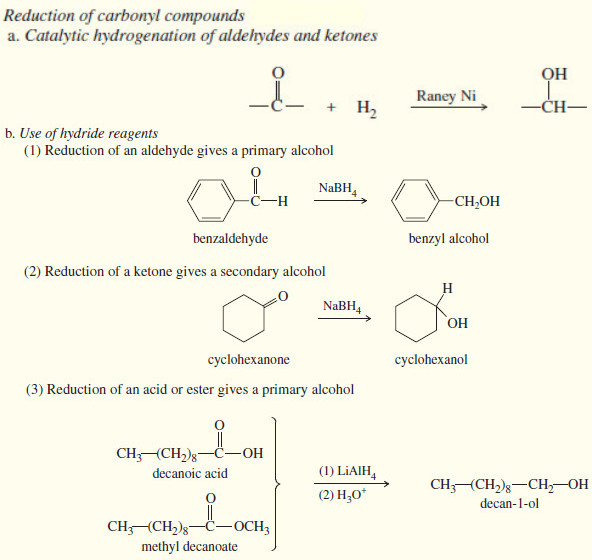
Reduction of the Carbonyl group : Synthesis of Alcohols
Reduction of the Carbonyl group : Synthesis of 1° and 2° Alcohols – Grignard reagents convert carbonyl group to alcohols…
Read More » -
Physical Chemistry
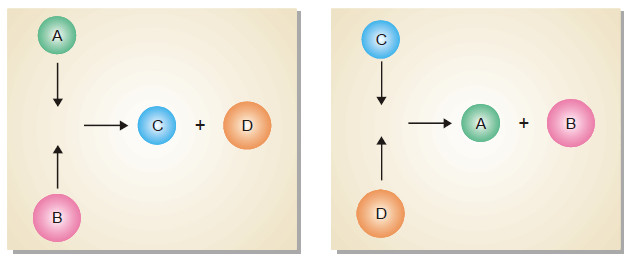
Characteristics of Chemical Equilibrium
Chemical Equilibrium is the state of a reversible reaction when the two opposing reactions occur at the same rate and…
Read More » -
Organic Chemistry
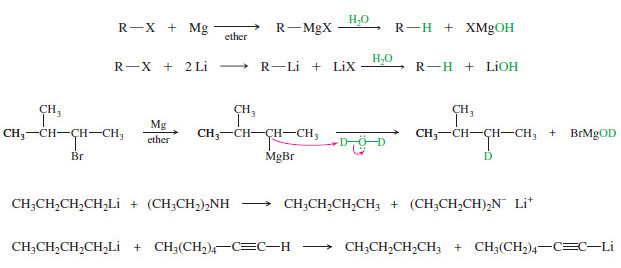
Side Reactions of Organometallic Reagents
Side Reactions of Organometallic Reagents: Reduction of Alkyl Halides – Organometallic Reagents: Grignard and organolithium reagents are strong nucleophiles and…
Read More » -
Organic Chemistry
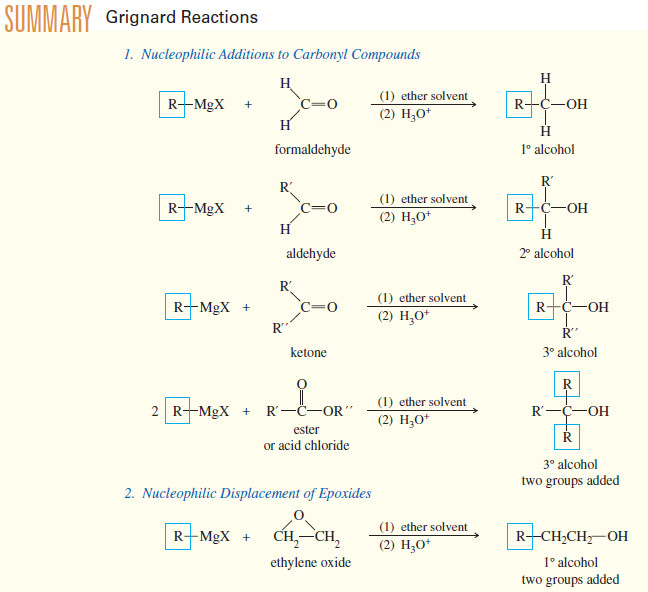
Addition of Grignard Reagents to Carbonyl Compounds
Addition of Organometallic Reagents to Carbonyl Compounds – Because they resemble carbanions, Grignard reagents and organolithium reagents are strong nucleophiles…
Read More »