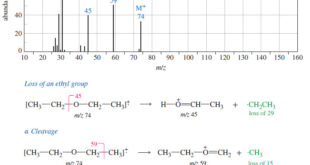
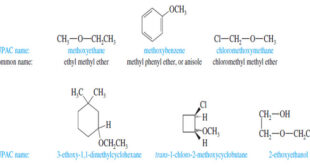
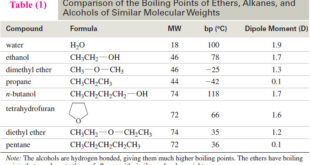
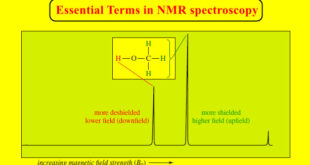
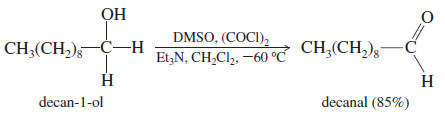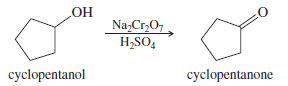Additional Methods for Oxidizing Alcohols
– Many other reagents and procedures have been developed for oxidizing alcohols.
– Some are simply modifications of the procedures we have seen.
– For example, the Collins reagent is a complex of chromium trioxide and pyridine, the original version of PCC.
– The Jones reagent is a milder form of chromic acid: a solution of diluted chromic acid in acetone.
– All of the chromium reagents produce by-products and washings that contain hazardous chromium salts and must be collected as hazardous waste.
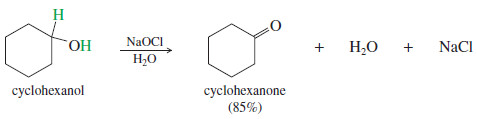
– In many cases, simple oxidants such as household bleach (sodium hypochlorite, NaOCl) can accomplish the same oxidations as chromic acid without using heavy metals, and without generating hazardous waste.
– Oxidations using sodium hypochlorite involve mildly acidic or basic conditions that may be better than chromic acid for acid sensitive compounds.
– Two other strong oxidants are potassium permanganate and nitric acid.
– Both of these reagents are less expensive than the chromium reagents, and both of them give by-products that are less environmentally hazardous than spent chromium reagents.
– Both permanganate and nitric acid oxidize secondary alcohols to ketones and primary alcohols to carboxylic acids.
– Used primarily in industry, these strong oxidants can form explosive mixtures and cleave carbon–carbon bonds if the temperature and concentrations are not precisely controlled.
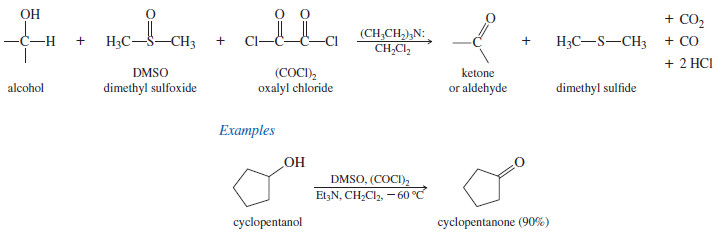
– The Swern oxidation uses dimethyl sulfoxide (DMSO) as the oxidizing agent to convert alcohols to ketones and aldehydes.
– DMSO and oxalyl chloride are added to the alcohol at low temperature, followed by a hindered base such as triethylamine.
– The reactive species (CH3)2 Cl, formed in the solution, is thought to act as the oxidant in the Swern oxidation.
– Secondary alcohols are oxidized to ketones, and primary alcohols are oxidized only as far as the aldehyde.
– The by-products of this reaction are all volatile and are easily separated from the organic products
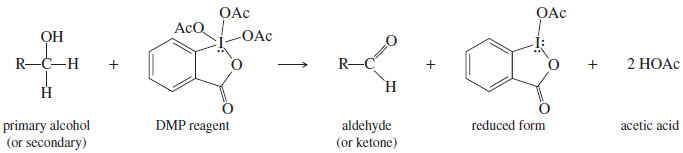
– Like the Swern oxidation, the Dess–Martin periodinane (DMP) reagent oxidizes primary alcohols to aldehydes and secondary alcohols to ketones without using chromium or other heavy-metal compounds.
– The reaction with DMP takes place under mild conditions (room temperature, neutral pH) and gives excellent yields.
– The DMP reagent, which owes its oxidizing ability to a high-valence iodine atom, is a commercially available solid that is easily stored
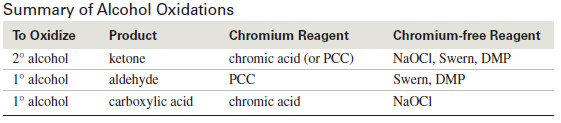
Summary of Alcohol Oxidations
Solved problem for Oxidizing Alcohols
Suggest the most appropriate method for each of the following laboratory syntheses:
(a) cyclopentanol → cyclopentanone
Solution:
– Many reagents are available to oxidize a simple secondary alcohol to a ketone.
– Most labs would have chromium trioxide or sodium dichromate available, and the chromic acid oxidation would be simple.
– Bleach (sodium hypochlorite) might be a cheaper and less polluting alternative to the chromium reagents.
– DMP and the Swern oxidation would also work.
(b) oct-2-en-1-ol oct-2-enal
Solution:
– This synthesis requires more finesse.
– The aldehyde is easily over-oxidized to a carboxylic acid, and the double bond reacts with oxidants such as Our choices are limited to PCC, DMP, or the Swern oxidation.
 Read Chemistry
Read Chemistry