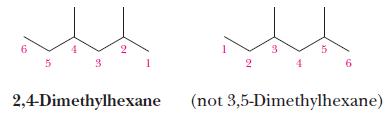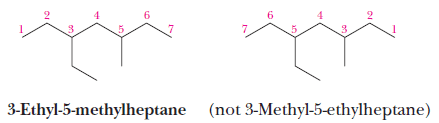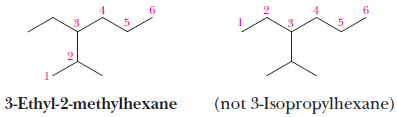Nomenclature of Alkanes: Rules, IUPAC Name, Common Name
– In this subject, we will discuss the Nomenclature of Alkanes: Rules, IUPAC Name, Common Name
What is Alkanes?
– Alkanes are saturated hydrocarbons; that is, they contain only carbon-carbon single bonds.
– In this context, saturated means that each carbon has the maximum number of hydrogens bonded to it.
Nomenclature of Alkanes: IUPAC Name
– Ideally, every organic compound should have a name from which its structural formula can be drawn.
– For this purpose, chemists have adopted a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC).
– The IUPAC name of an alkane with an unbranched chain of carbon atoms consists of two parts:
- The prefix indicates the number of carbon atoms in the chain.
- The suffix -ane shows that the compound is a saturated hydrocarbon.
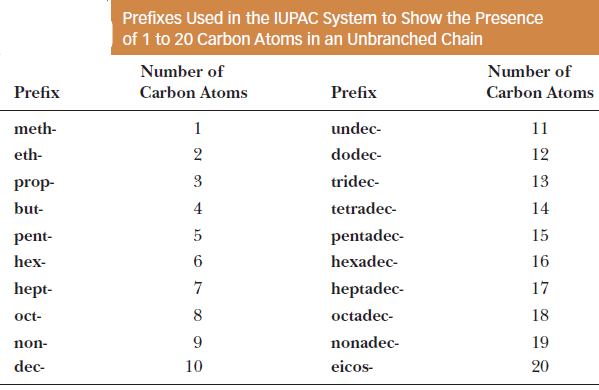
– The table gives the prefixes used to show the presence of 1 to 20 carbon atoms.
– The first four prefixes listed in the Table were chosen by the IUPAC because they were well-established in the language of organic chemistry.
– They were well established even before there were hints of the structural theory underlying the discipline.
– For example, the prefix but- appears in the name butyric acid, a compound of four carbon atoms formed by air oxidation of butter (Latin: butyrum, butter).
– Prefixes to show five or more carbons are derived from Greek or Latin numbers.
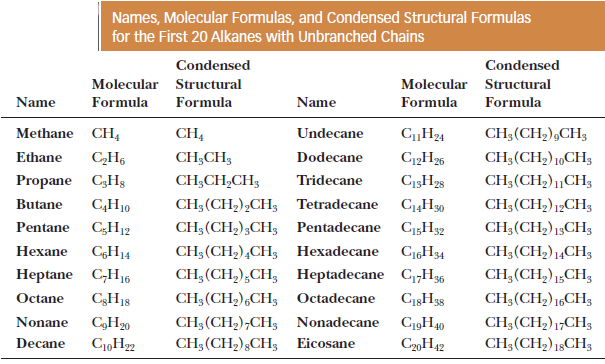
– The table below indicates for the names, molecular formulas, and condensed structural formulas for the first 20 unbranched alkanes.
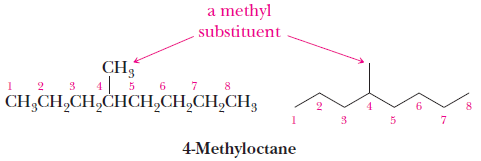
– The IUPAC name of an alkane with a branched chain consists of a parent name that indicates the longest chain of carbon atoms in the compound and substituent names that indicate the groups bonded to the parent chain.
Alkyl group
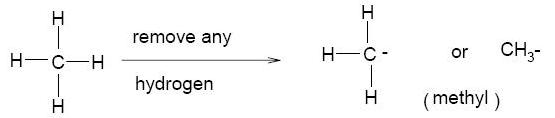
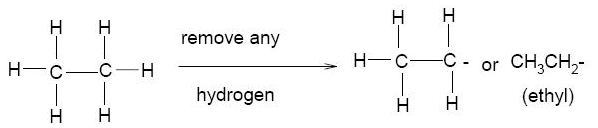
– The alkyl group is A substituent group derived from an alkane by the removal of a hydrogen atom.
– The alkyl group is represented by the symbol R-.
– We name alkyl groups by dropping the -ane from the name of the parent alkane and adding the suffix -yl.
– The substituent derived from methane, for example, is methyl, -CH3 and that derived from ethane is ethyl, CH3CH2–
Rules of the IUPAC system for naming alkanes:
(1) The name for an alkane with an unbranched chain of carbon atoms consists of a prefix showing the number of carbon atoms in the chain and the ending -ane.
(2) For branched-chain alkanes, select the longest chain of carbon atoms as the parent chain; its name becomes the root name.
(3) Give each substituent on the parent chain a name and a number.
– The number shows the carbon atom of the parent chain to which the substituent is bonded.
– Use a hyphen to connect the number to the name.
(4) If there is one substituent, number the parent chain from the end that gives the substituent the lower number.
(5) If there are two or more identical substituents, number the parent chain from the end that gives the lower number to the substituent encountered first.
– The number of times the substituent occurs is indicated by the prefix di-, tri-, tetra-, penta-, hexa-, and so on.
– A comma is used to separate position numbers.
(6) If there are two or more different substituents, list them in alphabetical order, and number the chain from the end that gives the lower number to the substituent encountered first.
– If there are different substituents in equivalent positions on opposite ends of the parent chain, give the substituent of lower alphabetical order the lower number.
(7) The prefixes di-, tri-, tetra-, and so on are not included in alphabetizing.
– Alphabetize the names of the substituents first, and then insert these prefixes.
– In the following example, the alphabetizing parts are ethyl and methyl, not ethyl and dimethyl.
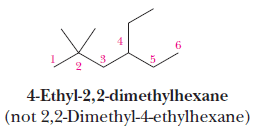
(8) Where there are two or more parent chains of identical length, choose the parent chain with the greater number of substituents.
Substituents of Alkanes
(1) Substituents are named following this same set of rules.
– Those with unbranched chains are named by dropping -ane from the name of the parent alkane and replacing it with -yl.
– Thus, unbranched alkyl substituents are named methyl, ethyl, propyl, butyl, pentyl, and so forth.
(2) Substituents with branched chains are named according to rules 2 and 3.
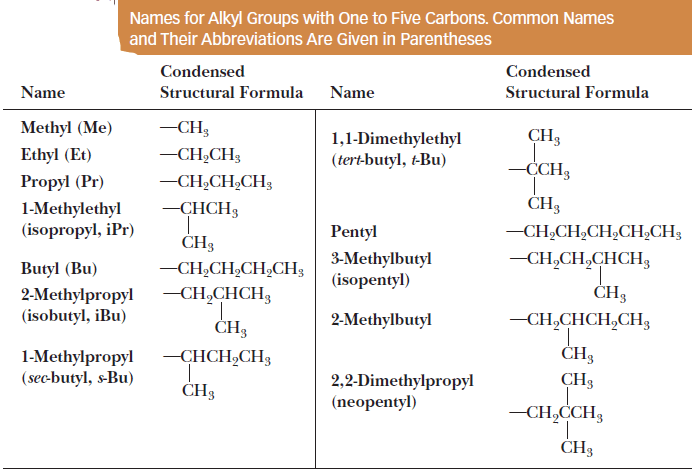
(3) The IUPAC names and structural formulas for unbranched and branched alkyl groups containing one to five carbon atoms are given in Table blow.
– Also given in parentheses are common names for the alkyl substituents.
(4) Their common names are so deeply entrenched in organic chemistry that in the official IUPAC nomenclature system, it is acceptable to use either the formal IUPAC name (such as 1-methylethyl) or the common name (in this case, isopropyl) for the alkyl substituents given in Table:
Nomenclature of Alkanes: Common Name
– In an alternative system known as common nomenclature, the total number of carbon atoms in an alkane, regardless of their arrangement, determines the name.
(1) The first three alkanes are methane, ethane, and propane.
(2) All alkanes with the molecular formula C4H10 are called butanes, all those with the molecular formula C5H12 are called pentanes, all those with the molecular formula C6H14 are called hexanes, and so forth.
(3) The fact that an alkane chain is unbranched is sometimes indicated by the prefix n- (normal); an example is n-pentane for CH3CH2CH2CH2CH3.
– For branched-chain alkanes beyond propane, iso- indicates that one end of an otherwise unbranched chain terminates in a (CH3)2CH- group, and neo- indicates that it terminates in -C(CH3)3.
(4) The following are examples of common names:
(5) This system of common names has no good way of naming other branching patterns; for more complex alkanes, it is necessary to use the more flexible IUPAC system of nomenclature.
Notes on Nomenclature of Alkanes
– We concentrate on IUPAC names. However, we also use common names, especially when the common name is used almost exclusively in everyday discussions among chemists.
– When both IUPAC and common names are given in the text, we give the IUPAC name first, followed by the common name in parentheses.
– In this way, you should have no doubt about which name is which.
Reference: Organic chemistry / William H. Brown, Christopher S. Foote, Brent L. Iverson, Eric V. Anslyn, Bruce M. Novak. ( sixth edition) . United States.