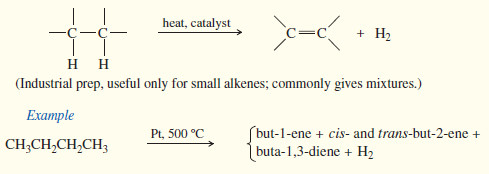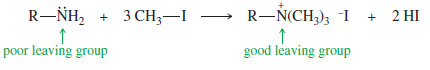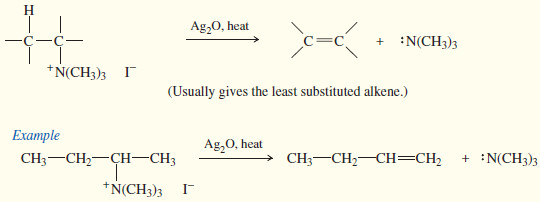Synthesis of Alkenes – Six methods
Methods for Synthesis of Alkenes
– Six methods for Synthesis of Alkenes will be discussed as follow:
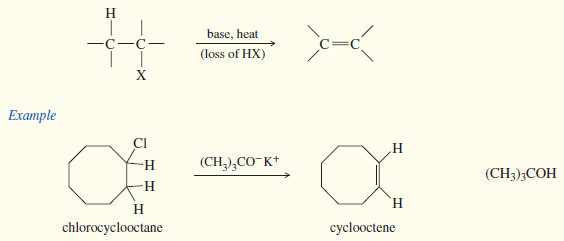
(1) Dehydrohalogenation of alkyl halides
– Dehydrohalogenation is the elimination of a hydrogen and a halogen from an alkyl halide to form an alkene.
– dehydrohalogenation can take place by the E1 and E2 mechanisms.
– The second-order elimination (E2) is usually better for synthetic purposes because the E1 has more competing reactions.
– Second-order elimination is a reliable synthetic reaction, especially if the alkyl halide is a poor SN2 substrate.
– E2 dehydrohalogenation takes place in one step, in which a strong base abstracts a proton from one carbon atom as the leaving group leaves the adjacent carbon
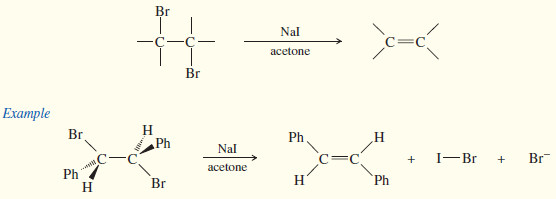
(2) Dehalogenation of vicinal dibromides
Debromination of Vicinal Dibromides
– Vicinal dibromides (two bromines on adjacent carbon atoms) are converted to alkenes by reduction with iodide ion in acetone.
– This debromination is rarely an important synthetic reaction, because the most likely origin of a vicinal dibromide is from bromination of an alkene.
– We discuss this reaction with dehydrohalogenation because the mechanisms are similar.
– Debromination is formally a reduction because a molecule of Br2 (an oxidizing agent) is removed.
– The reaction with iodide takes place by the E2 mechanism, with the same geometric constraints as the E2 dehydrohalogenation.
– Elimination usually takes place through an anti-coplanar arrangement, as shown in the following Mechanism.
– Acetone serves as a convenient solvent that dissolves most alkyl halides and sodium iodide.
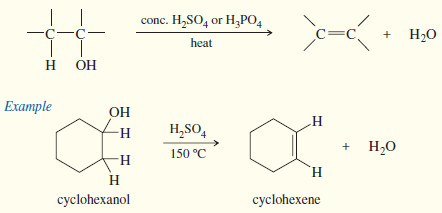
(3) Synthesis of Alkenes by Dehydration of alcohols
– Dehydration of alcohols is a common method for making alkenes. The word dehydration literally means “removal of water.”
– Dehydration is reversible, and in most cases the equilibrium constant is not large.
– In fact, the reverse reaction (hydration) is a method for converting alkenes to alcohols.
– Dehydration can be forced to completion by removing the products from the reaction mixture as they form.
– The alkene boils at a lower temperature than the alcohol because the alcohol is hydrogen bonded.
– A carefully controlled distillation removes the alkene while leaving the alcohol in the reaction mixture Concentrated sulfuric acid and/or concentrated phosphoric acid are often used as reagents for dehydration because these acids act both as acidic catalysts and as dehydrating agents.
– Hydration of these acids is strongly exothermic.
– The mechanism of dehydration resembles the E1 mechanism .
– The hydroxyl group of the alcohol is a poor leaving group (–OH), but protonation by the acidic catalyst converts it to a good leaving group (H2O).
– In the second step, loss of water from the protonated alcohol gives a carbocation.
– The carbocation is a very strong acid: Any weak base such as (H2O) or HSO4– can abstract the proton in the final step to give the alkene
(4) Dehydrogenation of alkanes
– Dehydrogenation is the removal of H2 from a molecule, just the reverse of hydrogenation.
– Dehydrogenation of an alkane gives an alkene.
– This reaction has an unfavorable enthalpy change but a favorable entropy change.
– The hydrogenation of alkenes is exothermic, with values of ΔH° around -80 to -120kj/mol (-20 to -30kj/mol ).
– Therefore, dehydrogenation is endothermic and has an unfavorable (positive) value of ΔH°
(5) Hofmann and Cope eliminations
– An amine cannot undergo elimination directly, however, because the leaving group would be an amide ion ( –NH2 or –NHR ), which is a very strong base and a poor leaving group.
– An amino group can be converted to a good leaving group by exhaustive methylation, which converts it to a quaternary ammonium salt that can leave as a neutral amine.
Exhaustive methylation is usually accomplished using methyl iodide.
Exhaustive methylation of an amine
– Elimination of the quaternary ammonium salt generally takes place by the E2 mechanism, which requires a strong base.
– To provide the base, the quaternary ammonium iodide is converted to the hydroxide salt by treatment with silver oxide.
– Heating of the quaternary ammonium hydroxide results in E2 elimination and formation of an alkene.
– This elimination of a quaternary ammonium hydroxide is called the Hofmann elimination.
(6) Synthesis of Alkenes by Reduction of alkynes
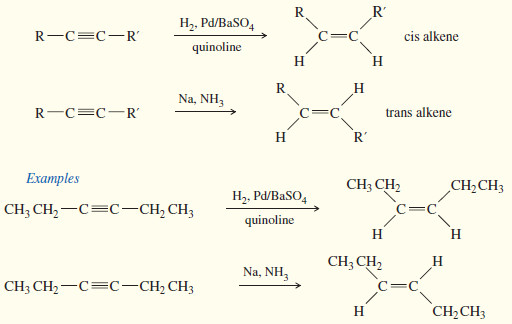
Catalytic Hydrogenation to cis Alkenes
– Hydrogenation of an alkyne can be stopped at the alkene stage by using a “poisoned” (partially deactivated) catalyst made by treating a good catalyst with a compound that makes the catalyst less effective.
– Lindlar’s catalyst is a poisoned palladium catalyst, composed of powdered barium sulfate coated with palladium, poisoned with quinoline.
– Nickel boride (Ni2B) is a newer alternative to Lindlar’s catalyst that is more easily made and often gives better yields
Metal–Ammonia Reduction to trans Alkenes
– To form a trans alkene, two hydrogens must be added to the alkyne with anti stereochemistry.
– Sodium metal in liquid ammonia reduces alkynes with anti stereochemistry, so this reduction is used to convert alkynes to trans alkenes
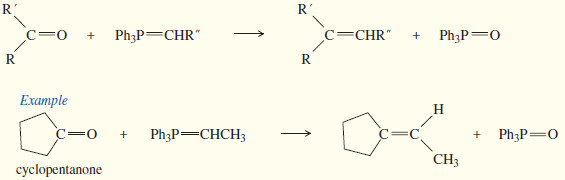
(7) Synthesis of Alkenes by Wittig reaction
– In 1954, Georg Wittig discovered a way of adding a phosphorus stabilized carbanion to a ketone or aldehyde.
– The product is not an alcohol, however, because the intermediate undergoes elimination to an alkene.
– In effect, the Wittig reaction converts the carbonyl group of a ketone or an aldehyde into a new double bond where no bond existed before.
– This reaction proved so useful that Wittig received the Nobel Prize in Chemistry in 1979 for this discovery