How to interpret IR spectrum without Knowledge of the structure
– In this subject, we will discuss How to interpret an IR spectrum without any Knowledge of the structure
How to interpret IR Spectrum without any Knowledge of the structure
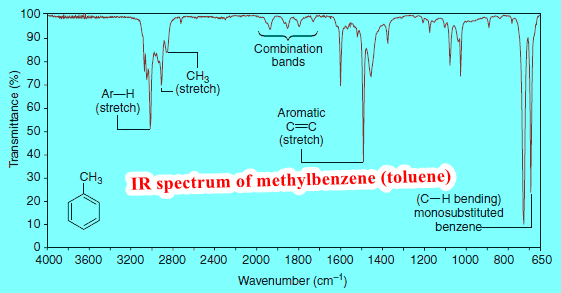
– IR spectroscopy is an incredibly powerful tool for functional group identification, as we have seen in the preceding subjects.
– To understand well, you should read these subjects in our site:
- Infrared Spectroscopy: An Instrumental Method for Detecting Functional Groups
- Interpreting IR Spectra
– However, in introducing this technique, we have explored IR spectra from the perspective of compounds of known structure, explaining the peaks observed about each critical grouping of atoms that we know to be present.
– In the real world, one often encounters brand-new materials of unknown structure.
– How IR can help in this scenario is something that a forensics scientist or natural products isolation chemist might need to worry about daily.
– We certainly cannot use IR spectroscopy by itself to determine the complete structure, but an IR spectrum can often point toward the presence of certain functional groups if one pays particular attention to signals whose peak positions are distinct from other groups and are consistently strong enough to be observed.
– The latter is an important consideration as there can be variations in signal strength for certain groups depending on what other groups are in the molecule, and some signals overlap with others, making a definitive assignment impossible.
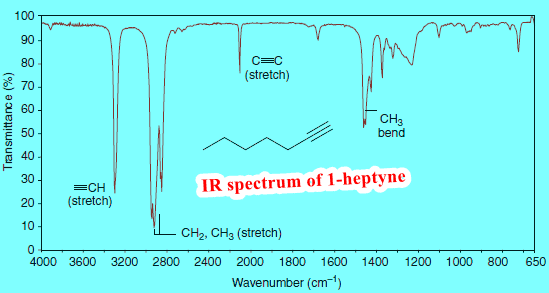
– For example, most organic molecules contain C-H bonds in one form or another, so peaks below 1450 cm-1 and signals in the range 2800–3000 cm-1 are not particularly definitive other than to indicate that the molecule is organic and contains C-H bonds.
Important Notes on the IR Spectrum
– Here are some examples of what one might consider in a first-pass assessment of any IR spectrum to generate what are likely to be correct answers about some of the functional groups that are present:
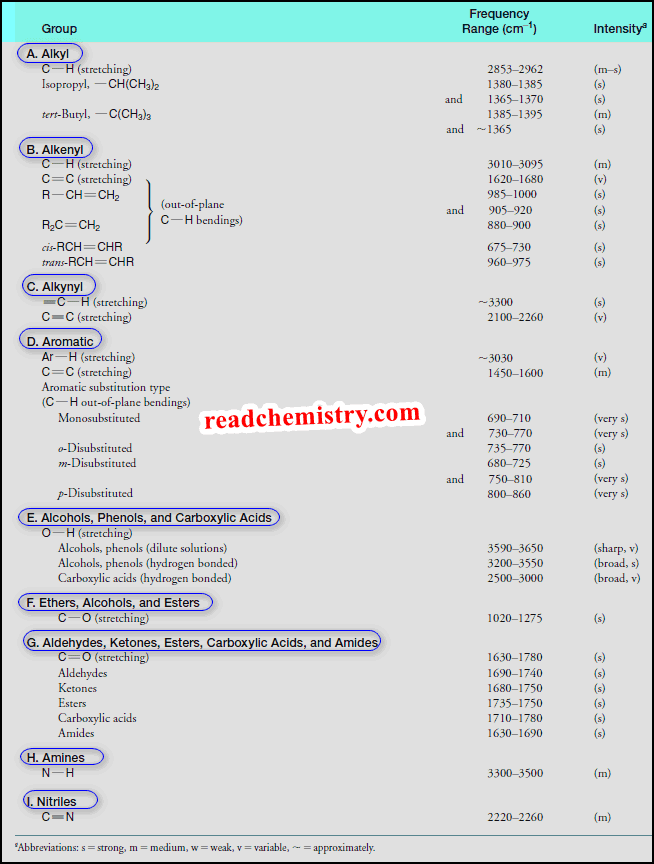
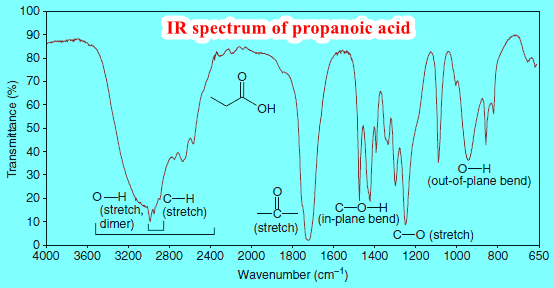
(1) Only carbonyl group (C=O) stretches tend to have a tight, strong absorbance in the 1630–1780 cm-1 range.
– We may not be able to identify what kind of carbonyl group is present, but we can tell that there is at least one carbonyl group.
(2) Only the stretches of nitrile or alkyne bonds ( C≡N, C≡C ) tend to appear between 2000 and 2300 cm-1, so these can be fairly readily assigned.
(3) Only hydroxyl groups (OH) as in alcohols or carboxylic acids (COOH) tend to create a large and broad signal at about 3300 cm-1; these groups are easy to identify assuming the sample is not contaminated with water.
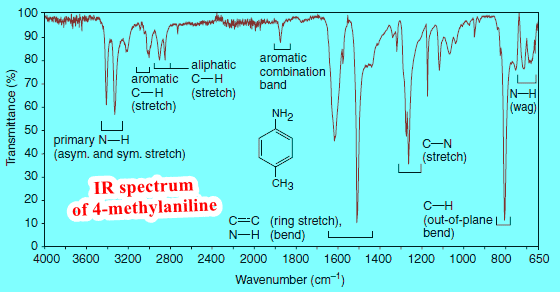
(4) Only amines tend to produce broad but smaller peaks than hydroxyl peaks around 3300 cm -1. The number of those peaks can sometimes tell if there is one or two hydrogens attached to that nitrogen atom.
Examples of IR spectrum
– The examples below allow us to put these general principles into practice.
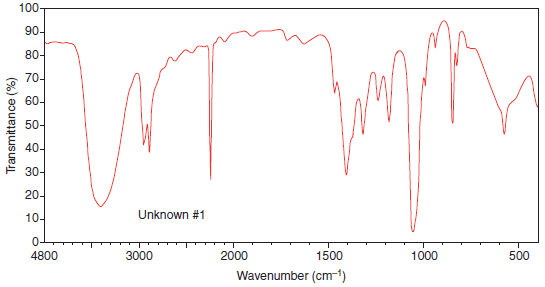
Example (1): The IR spectrum of Unknown (1)
(a) IR spectrum of Unknown (1) has broad signals centered around 3300 cm-1 and a medium absorption at 2250 cm-1.
– Based on the information above, we can surmise that the molecule likely contains a hydroxyl group and a group with a triple bond.
(b) Most likely the triply-bonded group is a nitrile since nitriles tend to appear at about 2250 cm-1, whereas alkynes appear slightly lower at around 2000 cm-1.
(c) We cannot be strictly sure that it is a nitrile, but that would be a good hypothesis in the absence of any other chemical evidence. Indeed, this turns out to be correct, as the molecule is 3-hydroxypropionitrile in this case.
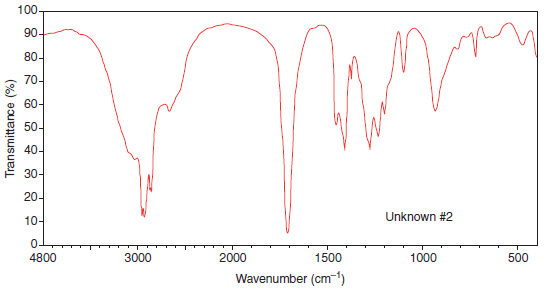
Example (2): The IR spectrum of Unknown (2)
(a) There is a hydroxyl absorption once again centered around 3300 cm-1, as well as a carbonyl peak at 1705 cm-1.
(b) And, although we cannot always tell what kind of carbonyl is present when the hydroxyl peak is extremely broad and has a ragged appearance (due to overlap of the C-H absorptions that extend below it, in contrast to the first spectrum where the hydroxyl was smooth, it is usually safe to assume that this hydroxyl group is attached to the carbonyl group; thus, these two groups are together part of a carboxylic acid functional group.
(c) Once again, we were able to identify the key functional group of the molecule since this is heptanoic acid.
Characteristic Infrared Absorptions of Groups
Solved problems
Reference: Organic chemistry / T.W. Graham Solomons , Craig B.Fryhle , Scott A.snyder , / ( eleventh edition) / 2014.