Quantum Numbers
– Bohr’s electronic energy shells or levels, designated as Principal Quantum Numbers (n), could hardly explain the hydrogen spectrum adequately.
– Spectra of other elements that are quite complex, also remained unexplained by this concept.
– Many single lines of the spectra are found to consist of a number of closely related lines when studied with the help of sophisticated instruments of high resolving power.
– Also the spectral lines split up when the source of radiation is placed in a magnetic field (Zeeman Effect) or in an electrical field (Stark Effect).
– To explain these facts, it is necessary to increase the number of (possible orbits) where an electron can be said to exist within an atom.
– In other words, it is necessary to allow more possible energy changes within an atom (or a larger number of energy states) to account for the existence of a larger number of such observed spectral lines.
– Wave mechanics makes a provision for three more states of an electron in addition to the one proposed by Bohr.
– Like the energy states of Bohr, designated by n = 1, 2, 3…, these states are also identified by numbers and specify the position and energy of the electron.
– Thus there are in all four such identification numbers called quantum numbers which fully describe an electron in an atom.
Types of Quantum Numbers
– There are four quantum numbers :
(1) Principal Quantum Number (n)
(2) Azimuthal Quantum number (l)
(3) Magnetic Quantum Number (m)
(4) Spin Quantum Number (s)
– Each one of these refers to a particular character.
(1) Principal Quantum Number (n)
– This quantum number denotes the principal shell to which the electron belongs.
– This is also referred to as major energy level.
– It represents the average size of the electron cloud i.e., the average distance of the electron from the nucleus.
– This is, therefore, the main factor that determines the values of nucleus-electron attraction, or the energy of the electron.
– In our earlier discussion, we have found that the energy of the electron and its distance from the nucleus for the hydrogen atom are given by:

where n is the principal quantum number of the shell.
– The principal quantum number (n) can have non-zero, positive, integral values n = 1, 2, 3… increasing by integral numbers to infinity.
– Although the quantum number (n) may theoretically assume any integral value from 1 to ∝ , only values from 1 to 7 have so far been established for the atoms of the known elements in their ground states.
– In a poly electron atom or ion, the electron that has a higher principal quantum number is at a higher energy level. An electron with n = 1 has the lowest energy and is bound most firmly to the nucleus.
– The letters K, L, M, N, O, P, and Q are also used to designate the energy levels or shells of electrons with a n value of 1, 2, 3, 4, 5, 6, and 7 respectively.
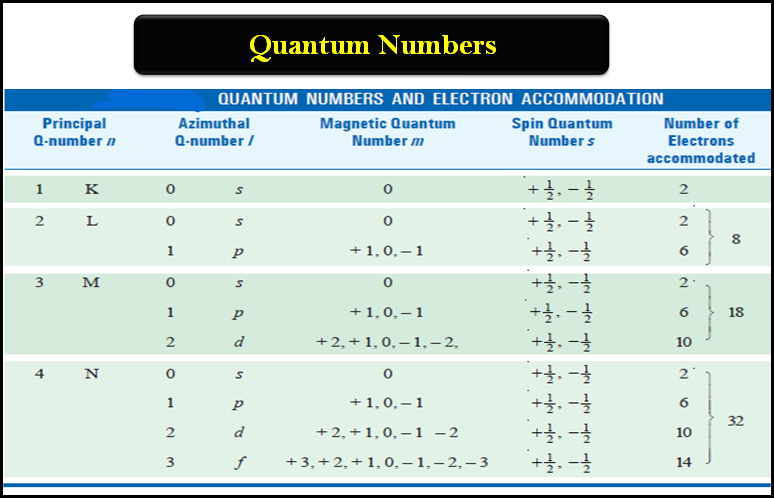
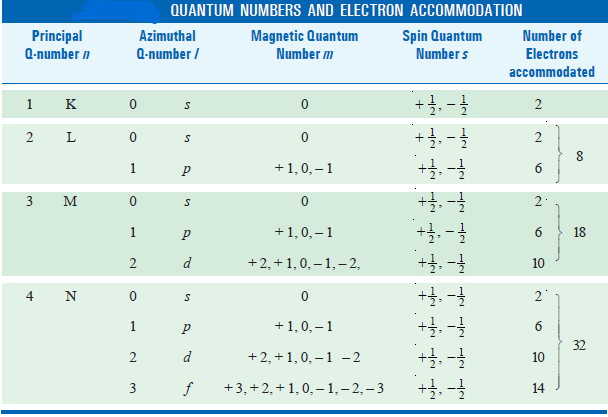
– There is a limited number of electrons in an atom that can have the same principal quantum number and is given by 2n2, where n is the principal quantum number concerned. Thus:
(2) Azimuthal Quantum number (l )
– This is also called a secondary or subsidiary quantum number.
– It defines the spatial distribution of the electron cloud about the nucleus and describes the angular momentum of the electron.
– The quantum number (l) defines the shape of the orbital occupied by the electron and the angular momentum of the electron.
– It is for this reason that (l) is sometimes referred to as an orbital or angular quantum number.
– For any given value of the principal quantum number n, the azimuthal quantum number (l) may have all integral values from (0) to (n – 1), each of which refers to an Energy sublevel or Sub-shell.
– The total number of such possible sublevels in each principal level is numerically equal to the principal quantum number of the level under consideration.
– These sublevels are also symbolized by letters (s, p, d, f, etc).
Important Observations
(1) for the principal quantum number n = 1, the only possible value for (l) is (0) i.e., there is only one possible subshell i.e. s-subshell (n = 1, l= 0).
(2) when (n) = 2, there are two possible values of l, l = 0 and l = 2 – 1 = 1.
This means that there are two subshells in the second energy shell with n = 2. These subshells are designated as 2s and 2p. Similarly,
(3) when n = 3, (l) can have three values i.e. 0, 1 and 2. Thus there are three subshells in third energy shell with designations 3s, 3p, and 3d respectively.
(4) when n = 4, there are four possible values of azimuthal quantum number l (= 0, 1, 2, and 3) each representing a different sublevel. In other words, the fourth energy level consists of four subshells which are designated as 4s, 4p, 4d, and 4f.
(5) Thus for different values of principal quantum numbers we have:
– For a given value of the principal quantum number, the order of increasing energy for different subshells is:
(3) Magnetic Quantum Number (m)
– This quantum number has been proposed to account for the splitting up of spectral lines (Zeeman Effect).
– An application of a strong magnetic field to an atom reveals that electrons with the same values of principal quantum number (n) and of azimuthal quantum number (l), may still differ in their behavior.
– They must, therefore, be differentiated by introducing a new quantum number, the magnetic quantum number (m).
– This is also called the Orientation Quantum Number because it gives the orientation or distribution of the electron cloud.
– For each value of the azimuthal quantum number (l), the magnetic quantum number (m), may assume all the integral values between + l to – l through zero i.e., + l, (+ l – l),… 0…, (– l + 1), – l.
– Therefore for each value of (l) there will be (2l + 1) values of ml.
When l = 0 (S orbital)
– when l = 0, m = 0 and no other value.
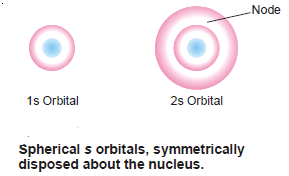
– This means that for each value of principal quantum number (n), there is only one orientation for l = 0 (s orbital) or there is only one (s orbital).
– For (s) orbital, there being only one orientation, it must be spherically symmetrical about the nucleus.
– There is only one spherically symmetrical orbital for each value of (n) whose radius depends upon the value of (n).
When l = 1 (p orbital)
– For l = 1 (p orbital), the magnetic quantum number (m) will have three values: + 1, 0, and – 1; so there are three orientations for p orbitals.
– These three types of p orbitals differ only in the value of magnetic quantum number and are designated as px, py, and pz depending upon the axis of orientation. The subscripts x, y, and z refer to the coordinate axes.
– In the absence of a magnetic field, these three p orbitals are equivalent in energy and are said to be three-fold degenerate or triply degenerate (Different orbitals of equivalent energy are called degenerate orbitals and are grouped together).
– In the presence of an external magnetic field the relative energies of the three p orbitals vary depending upon their orientation or magnetic quantum number.
– This probably accounts for the existence of more spectral lines under the influence of an external magnetic field.
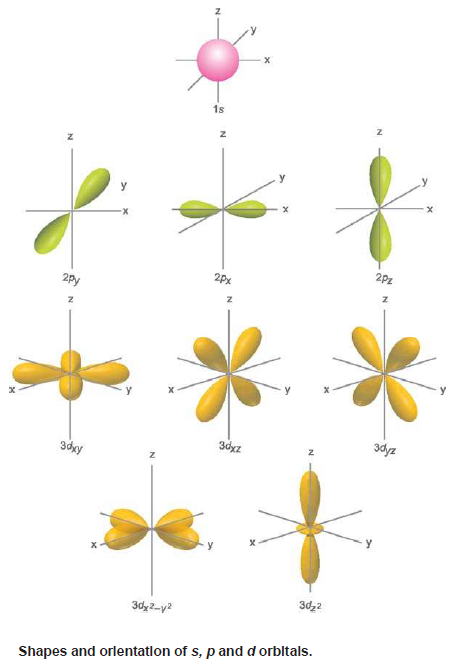
– The p orbital is of dumb-bell shape consisting of two lobes.
– The two lobes of a p orbital extend outwards and away from the nucleus along the axial line.
– Thus the two lobes of a p orbital may be separated by a plane that contains the nucleus and is perpendicular to the corresponding axis. Such a plane is called a nodal plane.
– There is no likelihood of finding the electron on this plane.
– For a px orbital, the yz plane is the nodal plane.
– The shapes and orientations of the p orbitals are given in Fig (1):
When l = 2 (d orbital)
– For l = 2 (d orbital), the magnetic quantum number is five (2 × 2 + 1); + 2, + 1, 0, – 1, – 2.
– Thus there are five possible orientations for d orbitals which are equivalent in energy so long as the atom is not under the influence of a magnetic field and are said to be five-fold degenerate (Different orbitals of equivalent energy are called degenerate orbitals and are grouped together).
– The five d orbitals are designated as dxy, dyz, dzx, dx2– y2 , dz2. These orbitals have complex geometrical shapes as compared to p orbitals.
– The conventional boundary surfaces or shapes of five dz2 orbitals are shown in Fig(1).
– The shape of the dz orbitals is different from others.
When l = 3 (f orbital)
– When l = 3 (f orbital) the magnetic quantum number m can have seven (2 × 3 + 1) values as + 3, + 2, + 1, 0, – 1, – 2, and – 3.
– These seven orientations give rise to a set of seven-fold degenerate orbitals.
– These seven orbitals possess very complicated shapes and orientation in space.
– The shapes of s, p, and d orbitals only are of interest to chemists.
(4) Spin Quantum Numbers (s)
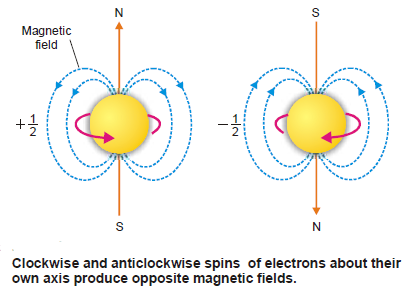
– This quantum number has been introduced to account for the spin of electrons about their own axis.
– Since an electron can spin clockwise or anticlockwise (in two opposite directions), there are two possible values of s that are equal and opposite.
– As quantum numbers can differ only by unity from each other, there are two values given to s; +½ and –½ depending upon whether the electron spins in one direction or the other.
– These spins are also designated by arrows pointing upwards and downward as ↓↑.
– Two electrons with the same sign of the spin quantum numbers are said to have parallel spins while those having opposite signs of the spin quantum numbers are said to have opposite spin or antiparallel spin or paired-up spin.
– Since a spinning charge is associated with a magnetic field, an electron must have a magnetic moment associated with it.
– The permitted values for each of these quantum numbers are given in the following table:
Solved Problem on Quantum Numbers
Problem (1): List all possible values of l and m for n = 2.
Solution:
– Here, the principal quantum number n = 2.
– The azimuthal quantum number can have only two values. These are 0 and 1
Problem (2): Which of the following sets of quantum numbers are not allowable and why?
Solution:
(a) Not allowable as l cannot have value equal to 2 when n = 2.
(b) Allowable
(c) Not allowable as l cannot have value equal to 1 when n = 1
(d) Not allowable as s cannot have value equal to 0.
(e) Allowable
Problem (3): What designations are given to the orbitals having
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.
 Read Chemistry
Read Chemistry