Pauli Exclusion Principle
– The Pauli exclusion principle is of immense value in telling the maximum number of electrons accommodated in any shell.
Quantum Numbers and the Energy of an Orbital
– The nature of an electron, its position, and energy, is fully implied only by mentioning the values of four quantum numbers ascribed to it.
– Each electron is, therefore, fully characterized by a set of four quantum numbers (n) – giving the size of the electron orbital, (l) – its shape, and (m) – the orientation or disposition of the orbital, and (s) the spin of the electron.
– Electrons having the same value of (n), the principal quantum number, are said to belong to the same major energy level.
– However, the energies possessed by these electrons may yet be different owing to the different values of other quantum numbers assigned to them.
– In fact, the major energy levels are made of sublevels, given by the value of azimuthal quantum number (l).
– A particular energy sublevel may be designated by s, p, d, and f.
– Within each energy level, the various sublevels have slightly different energies which increase in the same order as the value of the azimuthal quantum number (l).
– Therefore, for the major energy level n = 4, which has an (s) orbital (l = 0), p orbitals (l = l), d orbitals (l = 2), and f orbitals (l = 3), the energy increases in the order: s < p < d < f.
– An electron with the principal quantum number (n) and azimuthal quantum number (l) has always lesser energy than that of an electron with principal quantum number (n + 1) and the same azimuthal quantum number (l) i.e., the energy of a 3s orbital is less than that of 4s orbital and energy of 4p orbitals is always more than the energy of 3p orbitals, and so on.
– The other two quantum numbers namely magnetic and spin quantum numbers determine the maximum number of electrons that can be accommodated in orbitals of a sublevel.
– It is, therefore, the assignment of the four quantum numbers to the electrons which ultimately count to determine its energy and location in space within an atom.
Pauli Exclusion Principle
– Wolfgang Pauli put forward an ingenious principle that controls the assignment of values of four quantum numbers of an electron.
– It applies certain restrictions on the values of electrons in an atom and hence the name (exclusion principle).
– The Pauli exclusion principle is stated as: No two electrons in an atom can have the same set of four identical quantum numbers.
– Even if two electrons have the same values for n, l, and m, they must have different values of (s).
– Thus every electron in an atom differs from every other electron in total energy and, therefore, there can be as many electrons in a shell as there are possible arrangements of different quantum numbers.
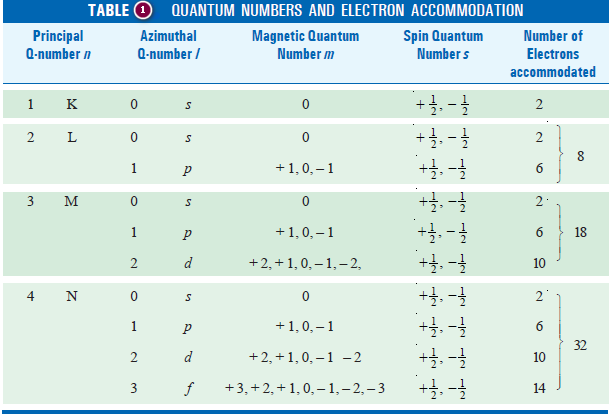
– The arrangements of electrons using permitted quantum numbers n, l, m, and s are given in the following table (1):
The maximum number of electrons in an orbital
– Let us find out the maximum number of electrons that can be accommodated in an orbital.
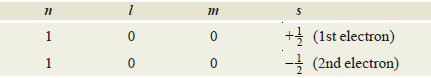
– We have seen that the first shell (n = 1) has only one orbital i.e., 1s.
– The possible arrangements for the quantum numbers are only two in accordance with Pauli’s exclusion principle.
– It follows, therefore, that a maximum of two electrons can be accommodated in an orbital and they must possess opposite spins.
– Consider the second shell (n = 2), there being four orbitals, one s orbital (l = 0) and three p orbitals (l = 1), the possible number of electrons having different set of quantum numbers can be as follows :
– The total number of electrons that can be accommodated in the second shell is equal to 2 + 6 = 8.
– Similarly, it can be shown that the maximum number of electrons in the third and fourth shells is equal to 18 and 32 respectively.
– On the basis of the above direction and Table(1) it follows that s sublevel may contain up to two electrons, (p) sublevel up to six, (d) sublevel up to ten, and (f) sublevel may have up to fourteen electrons.
– Each sublevel can accommodate at the most twice the number of available orbitals at that sublevel.
– The Pauli exclusion principle is of immense value in telling the maximum number of electrons accommodated in any shell.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.