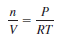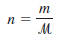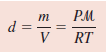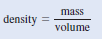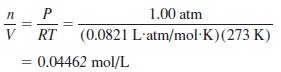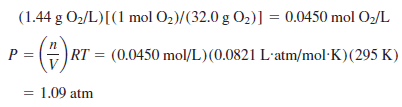Density of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Density of gas (Definition, Equation, Solved Examples)
The Density of gas
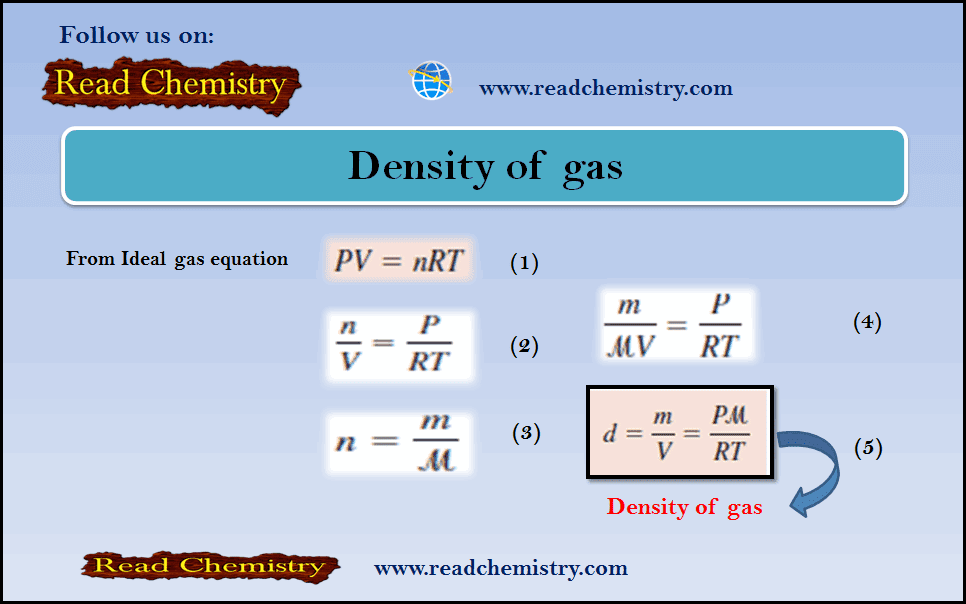
– From the ideal gas equation:
– If we rearrange the ideal gas equation, we can calculate the density of a gas:
– The number of moles of the gas, n, is given by:
– where m is the mass of the gas in grams and µ is its molar mass. Therefore
– Because density, d, is mass per unit volume, we can write:
– Unlike molecules in condensed matter (that is, in liquids and solids), gaseous molecules are separated by distances that are large compared with their size.
– Consequently, the density of gases is very low under atmospheric conditions.
– For this reason, gas densities are usually expressed in grams per liter (g/L) rather than grams per milliliter (g/mL).
Solved problems on the Density of gas
Problem (1)
Calculate the density of carbon dioxide (CO2) in grams per liter (g/L) at 0.990 atm and 55oC.
Strategy:
– We need the combined gas Equation to calculate gas density.
– Is sufficient information provided in the problem? What temperature unit should be used?
Solution:
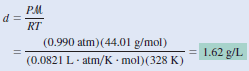
– To use the combined gas Equation, we convert temperature to kelvins ( T = 273 + 55 = 328 K) and use 44.01 g for the molar mass of CO2:
– Alternatively, we can solve for the density by writing:
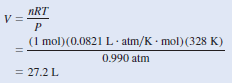
– Assuming that we have 1 mole of CO2, the mass is 44.01 g.
– The volume of the gas can be obtained from the ideal gas equation
– Therefore, the density of CO2 is given by:
Comment:
– ln units of grams per milliliter, the gas density is 1.62 × 10-3 g/mL, which is a very small number.
– In comparison, the density of water is 1.0 g/mL and that of gold is 19.3 g/cm3.
Problem (2)
Determine the density of nitrogen at STP.
Solution:
– It is easy to calculate the number of moles of nitrogen gas per liter using the ideal gas law:
– The density, the number of grams per liter, is obtained using the molar mass as a conversion factor:
Problem (3)
Calculate the pressure at at 22 oC which oxygen has a density of 1.44 g/L.
Solution:
– We first change the density in grams per liter to moles per liter, then use that value in the ideal gas law:
Reference:
- Chemistry / Raymond Chang, Williams College /(10th edition).
- Fundamentals of Chemistry / David E.Goldberg/(5th edition).