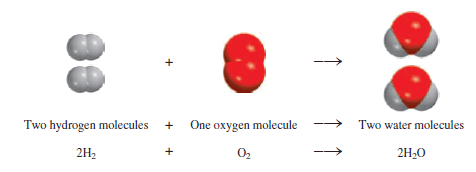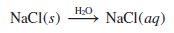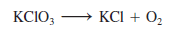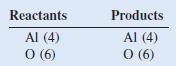Chemical Equations – Writing and Balancing Chemical Equations
– In this subject, we will discuss Writing and Balancing Chemical Equations.
Chemical Reactions and Chemical Equations
– A chemical reaction is a process in which a substance (or substances) is changed into one or more new substances.
– To communicate with one another about chemical reactions, chemists have devised a standard way to represent them using chemical equations.
– Chemical equations use chemical symbols to show what happens during a chemical reaction.
– In this subject, we will learn how to write chemical equations and balance them.
Writing Chemical Equations
Reaction between Hydrogen and Oxygen
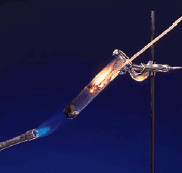
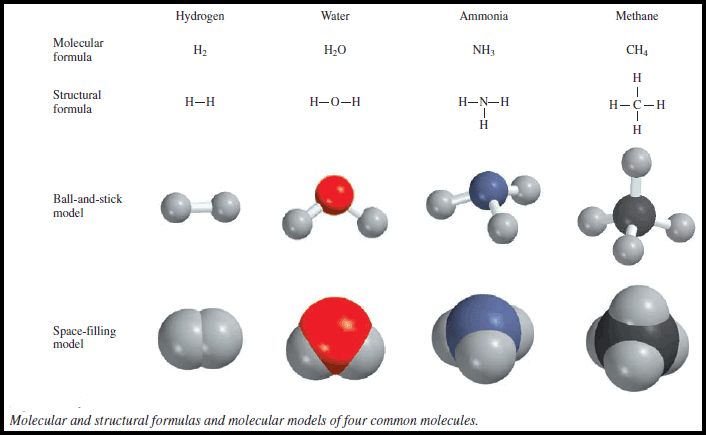
– Consider what happens when hydrogen gas (H2) burns in air (which contains oxygen, O2) to form water (H2O). This reaction can be represented by the chemical equation.
– where the “plus” sign means “reacts with” and the arrow means “to yield.”
– Thus, this symbolic expression can be read: “Molecular hydrogen reacts with molecular oxygen to yield water.”
– The reaction is assumed to proceed from left to right as the arrow indicates.
– Equation (1) is not complete, however, because there are twice as many oxygen atoms on the left side of the arrow (two) as on the right side (one).
– To conform with the law of conservation of mass, there must be the same number of each type of atom on both sides of the arrow; that is, we must have as many atoms after the reaction ends as we did before it started.
– We can balance Equation (1) by placing the appropriate coefficient (2 in this case) in front of H2 and H2O:
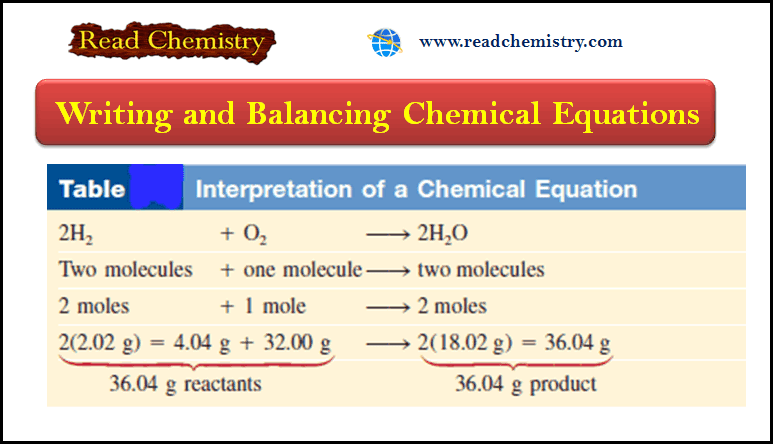
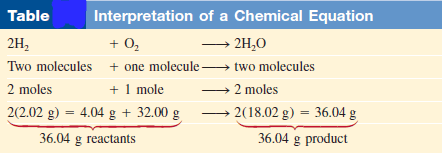
– This balanced chemical equation shows that “two hydrogen molecules can combine or react with one oxygen molecule to form two water molecules” (Figure).
– Because the ratio of the number of molecules is equal to the ratio of the number of moles, the equation can also be read as (2 moles of hydrogen molecules react with 1 mole of oxygen molecules to produce 2 moles of water molecules.)
– We know the mass of a mole of each of these substances, so we can also interpret the equation as (4.04 g of H2 reacting with 32.00 g of O2 to give 36.04 g of H2O.)
– These three ways of reading the equation are summarized in the following Table:
Important Notes for Writing Chemical Equations
(1) Reactants are the starting materials in a chemical reaction.
– H2 and O2are Reactants in Equation (1)
(2) Products are the substance formed as a result of a chemical reaction.
– Water is the Product in Equation (1)
(3) A chemical equation is just the chemist’s shorthand description of a reaction.
(4) In a chemical equation the reactants are conventionally written on the left and the products on the right of the arrow:
(5) To provide additional information, chemists often indicate the physical states of the reactants and products by using the letters g, l, and s to denote gas, liquid, and solid, respectively.
– For example:
– To represent what happens when sodium chloride (NaCl) is added to water, we write
where (aq) denotes the aqueous (that is, water) environment.
– Writing H2O above the arrow symbolizes the physical process of dissolving a substance in water, although it is sometimes left out for simplicity
Balancing Chemical Equations
– Suppose we want to write an equation to describe a chemical reaction that we have just carried out in the laboratory. How should we go about doing it? Because we know the identities of the reactants, we can write their chemical formulas.
– The identities of products are more difficult to establish.
– For simple reactions, it is often possible to guess the product(s).
– For more complicated reactions involving three or more products, chemists may need to perform further tests to establish the presence of specific compounds.
– Once we have identified all the reactants and products and have written the correct formulas for them, we assemble them in the conventional sequence— reactants on the left separated by an arrow from products on the right.
– The equation written at this point is likely to be unbalanced; that is, the number of each type of atom on one side of the arrow differs from the number on the other side.
Rules for balancing chemical equation
– In general, we can balance a chemical equation by the following steps
(1) Identify all reactants and products and write their correct formulas on the left side and right side of the equation, respectively.
(2) Begin balancing the equation by trying different coefficients to make the number of atoms of each element the same on both sides of the equation.
– We can change the coefficients (the numbers preceding the formulas) but not the subscripts (the numbers within formulas).
– Changing the subscripts would change the identity of the substance.
– For example, 2NO2 means “two molecules of nitrogen dioxide,” but if we double the subscripts, we have N2O4, which is the formula of dinitrogen tetroxide, a completely different compound.
(3) First, look for elements that appear only once on each side of the equation with the same number of atoms on each side.
– The formulas containing these elements must have the same coefficient.
– Therefore, there is no need to adjust the coefficients of these elements at this point.
Next, look for elements that appear only once on each side of the equation but in unequal numbers of atoms. Balance these elements.
– Finally, balance elements that appear in two or more formulas on the same side of the equation.
(4) Check your balanced equation to be sure that you have the same total number of each type of atom on both sides of the equation arrow.
Examples of Writing and Balancing Chemical Equations
Example (1): Heating of Potassium Chlorate
– Let’s consider a specific example.
– In the laboratory, small amounts of oxygen gas can be prepared by heating potassium chlorate (KClO3).
– The products are oxygen gas (O2) and potassium chloride (KCl). From this information, we write:
(For simplicity, we omit the physical states of reactants and products.)
– All three elements (K, Cl, and O) appear only once on each side of the equation, but only for K and Cl do we have equal numbers of atoms on both sides.
– Thus, KClO3 and KCl must have the same coefficient.
– The next step is to make the number of O atoms the same on both sides of the equation.
– Because there are three O atoms on the left and two O atoms on the right of the equation, we can balance the O atoms by placing a 2 in front of KClO3 and a 3 in front of O2.
– Finally, we balance the K and Cl atoms by placing a 2 in front of KCl:
– As a final check, we can draw up a balance sheet for the reactants and products where the number in parentheses indicates the number of atoms of each element:
– Note that this equation could also be balanced with coefficients that are multiples of 2 (for KClO3), 2 (for KCl), and 3 (for O2); for example:
– However, it is common practice to use the simplest possible set of whole-number coefficients to balance the equation. Equation (2) conforms to this convention.
Example (2): Combustion of ethane
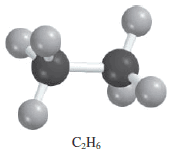
– Now let us consider the combustion (that is, burning) of the natural gas component ethane (C2H6) in oxygen or air, which yields carbon dioxide (CO2) and water.
– The unbalanced equation is:
– We see that the number of atoms is not the same on both sides of the equation for any of the elements (C, H, and O).
– In addition, C and H appear only once on each side of the equation; O appears in two compounds on the right side (CO2 and H2O).
– To balance the C atoms, we place a 2 in front of CO2:
– To balance the H atoms, we place a 3 in front of H2O:
– At this stage, the C and H atoms are balanced, but the O atoms are not because there are seven O atoms on the right-hand side and only two O atoms on the left-hand side of the equation.
– This inequality of O atoms can be eliminated by writing 7/2 in front of the O2 on the left-hand side:
– The “logic” for using 7/2 as a coefficient is that there were seven oxygen atoms on the right-hand side of the equation, but only a pair of oxygen atoms (O2) on the left.
– To balance them we ask how many pairs of oxygen atoms are needed to equal seven oxygen atoms.
– Just as 3.5 pairs of shoes equal seven shoes, 7/2 O2 molecules equal seven O atoms.
– As the following tally shows, the equation is now balanced:
– However, we normally prefer to express the coefficients as whole numbers rather than as fractions.
– Therefore, we multiply the entire equation by 2 to convert 7/2 to 7:
– The final tally is:
– Note that the coefficients used in balancing the last equation are the smallest possible set of whole numbers.
Example (3): Formation of aluminum oxide
– When aluminum metal is exposed to air, a protective layer of aluminum oxide (Al2O3) forms on its surface.
– The unbalanced equation is:
– In a balanced equation, the number and types of atoms on each side of the equation must be the same.
– We see that there is one Al atom on the reactants side and there are two Al atoms on the product side.
– We can balance the Al atoms by placing a coefficient of 2 in front of Al on the reactant’s side.
– There are two O atoms on the reactants side and three O atoms on the product side of the equation.
– We can balance the O atoms by placing a coefficient of 3/2 in front of O2 on the reactant side
– This is a balanced equation. However, equations are normally balanced with the smallest set of whole number coefficients.
– Multiplying both sides of the equation by 2 gives whole number coefficients
– Check For an equation to be balanced, the number and types of atoms on each side of the equation must be the same. The final tally is
– The equation is balanced.
Reference: General Chemistry: The Essential Concepts / Raymond Chang, Jason Overby. (sixth edition).