Nuclear Reaction: Definition, Types, Examples, Equations
– In this subject, we will discuss the Nuclear Reaction: Definition, Types, Examples, and Equations.
Nuclear Reaction
– In a chemical reaction, there is merely a rearrangement of extranuclear electrons.
– The atomic nucleus remains intact.
– A nuclear reaction involves a change in the composition of the nucleus.
– The number of protons and neutrons in the nucleus is altered.
– The product is a new nucleus of another atom with a different atomic number and/or mass number.
– Nuclear reaction: is one which proceeds with a change in the composition of the nucleus to produce an atom of a new element.
– The conversion of one element to another by a nuclear change is called Transmutation.
– We have already considered the nuclear reactions of radioactive nuclei, producing new isotopes.
– Here we will consider such reactions caused by artificial means.
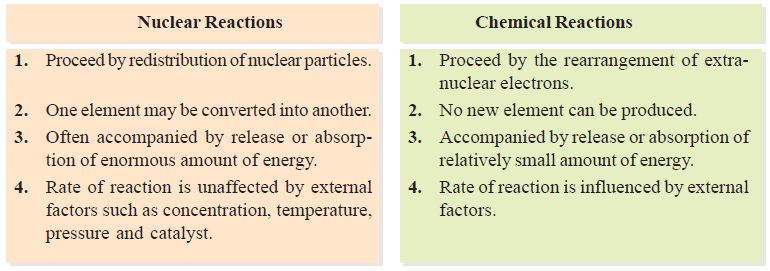
Differences between Nuclear Reaction and Chemical Reaction
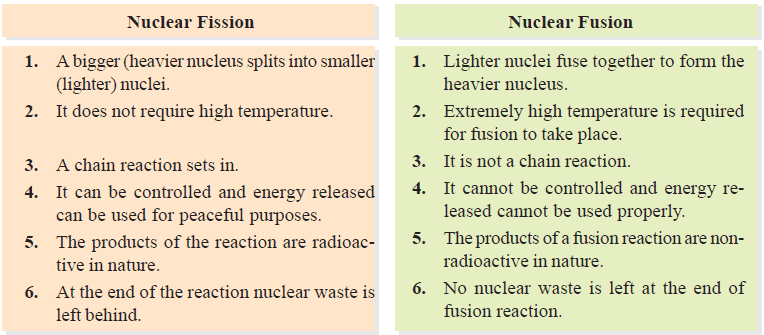
Nuclear Fission Reactions
– In these reactions, an atomic nucleus is broken or fissioned into two or more fragments.
– This is accomplished by bombarding an atom with alpha particles (4He2 ), neutrons (1n0), protons (1H1), deuterons (2H1), etc.
– All the positively charged particles are accelerated to high kinetic energies by a device such as a cyclotron.
– This does not apply to electrically neutral neutrons.
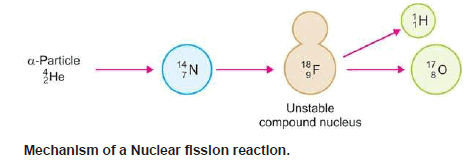
– The projectile enters the nucleus and produces an unstable ‘compound nucleus’.
– It decomposes instantaneously to give the products.
– For example, 14N7 when struck by an α-particle first forms an intermediate unstable compound nucleus, 18F9, which at once cleaves to form stables 17O8
– Other examples are:
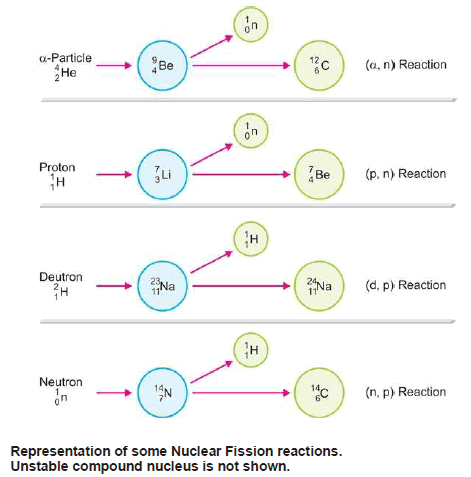
– Nuclear fission reactions are classified according to the projectile used and the particle that is emitted.
– In the Figure above the type of the reaction has been stated.
– It is noteworthy that neutrons are particularly useful as the projectile.
– Sir James Chadwick obtained these by bombarding beryllium-9 with α-particles.
– Being electrically neutral, neutrons pierce the positive nucleus easily.
Nuclear Fusion Reactions
– These reactions take place by combination or fusion of two small nuclei into a larger nucleus.
– At extremely high temperatures the kinetic energy of these nuclei overweighs the electrical repulsions between them.
– Thus they coalesce to give an unstable mass which decomposes to give a stable large nucleus and a small particle as the proton, neutron, positron, etc.
– For example :
(1) Two hydrogen nuclei, 1H1, fuse to produce a deuterium nucleus, 2H1.
(2) Deuterium nucleus, 2H1, and tritium nucleus, 3H1 , combine to give helium nucleus, 4He2 with the expulsion of a neutron.
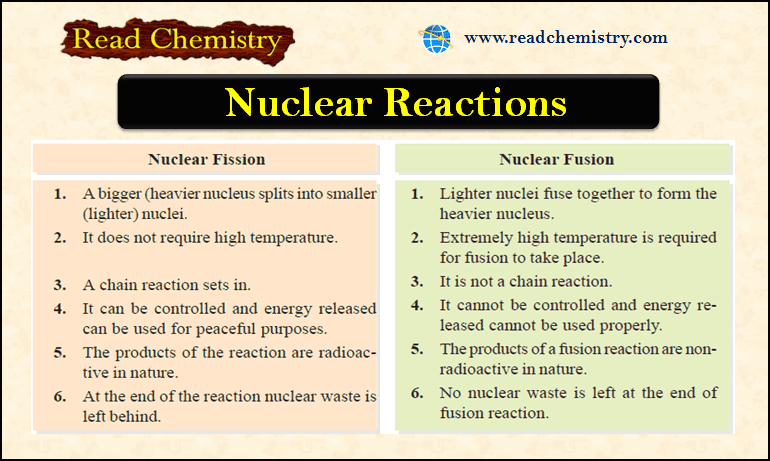
Differences between Nuclear Fission and Nuclear Fusion
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.



Very good