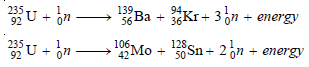Nuclear Fission: Definition, Properties, Examples, Applications
– In this subject, we will discuss the Nuclear Fission (Definition, Properties, Examples, Applications)
Nuclear Fission Process
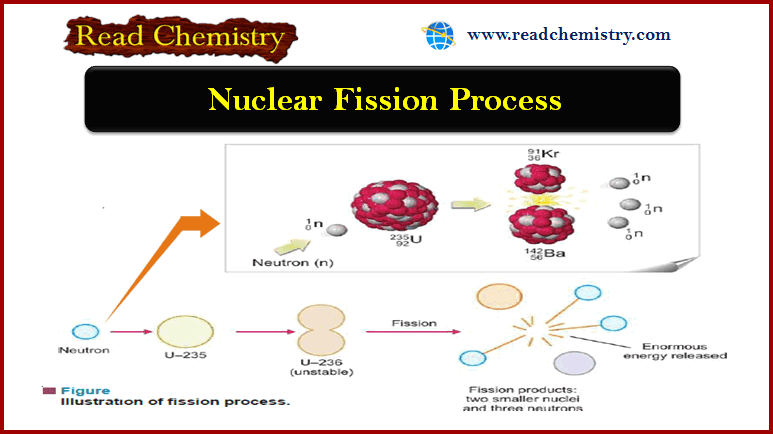
– In 1939, Hahn and Stassmann discovered that a heavy atomic nucleus of uranium-235 upon bombardment by a neutron splits apart into two or more nuclei.
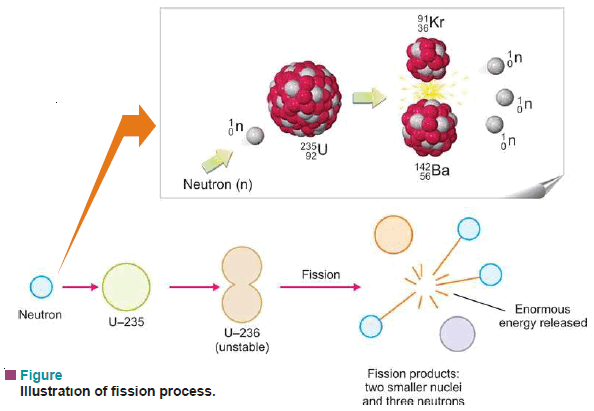
– U-235 first absorbs a neutron to form an unstable (compound nucleus).
– The excited ‘compound nucleus’ then divides into two daughter nuclei with the release of neutrons and a large amount of energy.
– The splitting of a heavy nucleus into two or more smaller nuclei is termed nuclear fission.
– The smaller nuclei formed as a result of fission are called fission products.
– The process of fission is always accompanied by the ejection of two or more neutrons and the liberation of vast energy.
– A given large nucleus can fission in many ways forming a variety of products.
– Thus the fission of U-235 occurs in about 35 ways.
– Two of these are given below in the form of equations:
– In these fission reactions, the mass of the products is less than the mass of the reactant.
– A loss of mass of about 0.2 amu per uranium atom occurs.
– This mass is converted into a fantastic quantity of energy which is 2.5 million times that produced by the equivalent amount of coal.
Properties of Nuclear fission
(1) Upon capturing a neutron, a heavy nucleus cleaves into two or more nuclei.
(2) Two or more neutrons are produced by fission of each nucleus.
(3) Vast quantities of energy are produced as a result of the conversion of small mass into energy.
(4) All the fission products are radioactive, giving off beta and gamma radiation.
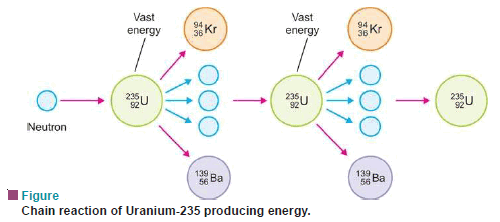
Nuclear Chain Reaction
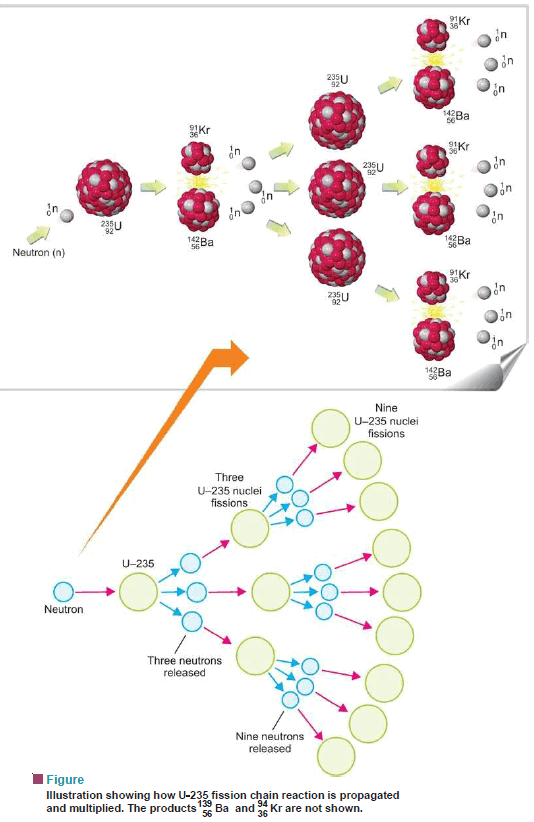
– We know that the U-235 nucleus when hit by a neutron undergoes the reaction:
– Each of the three neutrons produced in the reaction strikes another U-235 nucleus, thus causing nine subsequent reactions.
– These nine reactions, in turn, further give rise to twenty-seven reactions.
– This process of propagation of the reaction by multiplication in threes at each fission is referred to as a chain reaction.
– Heavy unstable isotopes, in general, exhibit a chain reaction by the release of two or three neutrons at each fission.
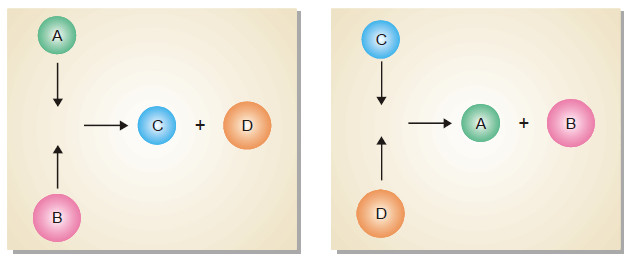
– It may be defined as A fission reaction where the neutrons from a previous step continue to propagate and repeat the reaction.
– A chain reaction continues till most of the original nuclei in the given sample are fissioned.
– However, it may be noted that not all the neutrons released in the reaction are used up in propagating the chain reaction. Some of these are lost to the surroundings.
– Thus for a chain reaction to occur, the sample of the fissionable material should be large enough to capture the neutron internally.
– If the sample is too small, most neutrons will escape from its surface, thereby breaking the chain.
– The minimum mass of fissionable material required to sustain a chain reaction is called critical mass.
– The critical mass varies for each reaction.
– For U-235 fission reaction, it is about 10 kg.
– As already stated, even a single fission reaction produces a large amount of energy.
– A chain reaction that consists of innumerable fission reactions will, therefore, generate many times greater energy.
Nuclear Energy
– A heavy isotope such as uranium-235 (or plutonium-239) can undergo nuclear chain reaction yielding vast amounts of energy.
– The energy released by the fission of nuclei is called nuclear fission energy or nuclear energy.
– Sometimes, it is incorrectly referred to as atomic energy.
– The fission of U-235 or Pu-239 occurs instantaneously, producing incomprehensible quantities of energy in the form of heat and radiation.
– If the reaction is uncontrolled, it is accompanied by explosive violence and can be used in atomic bombs.
– However, when controlled in a reactor, the fission of U-235 is harnessed to produce electricity.
First Chain Reaction
– The first controlled nuclear fission chain reaction, directed by Italian-born American physicist Enrico Fermi, is captured here in a painting of the event, which took place under the sports stadium at the University of Chicago in December 1942.
– The event was the forerunner of all nuclear reactors.
The Atomic Bomb
– A bomb that works on the principle of a fast nuclear chain reaction is referred to as an atomic bomb.
– The design of such a bomb is shown in Fig (1):
(1) It contains two subcritical masses of fissionable material, 235U or 239Pu.
(2) It has a mass of trinitrotoluene in a separate pocket.
(3) When TNT is detonated, it drives one mass of 235U into the other.
– A supercritical mass of the fissionable material is obtained.
(4) As a result of the instantaneous chain reaction, the bomb explodes with the release of tremendous heat energy.
The temperature developed in an atomic bomb is believed to be 10 million °C (the temperature of the sun).
– Besides many radio nuclei and heat, deadly gamma rays are released.
– These play havoc with life and the environment.
– If the bomb explodes near the ground, it raises tons of dust into the air.
– The radioactive material adhering to dust is known as fall out.
– It spreads over wide areas and is a lingering source of radioactive hazard for long periods
Little Boy And Fat Man
– Little Boy was the first nuclear weapon used in warfare.
– It exploded approximately 1,800 feet over Hiroshima, Japan, on the morning of August 6, 1945, with a force equal to 13,000 tons of TNT.
– Immediate deaths were between 70,000 to 130,000.
– Fat Man was the second nuclear weapon used in warfare.
– Dropped on Nagasaki, Japan, on August 9, 1945, Fat Man devastated more than two square miles of the city and caused approximately 45,000 immediate deaths.
– While Little Boy was a uranium gun-type device, Fat Man was a more complicated and powerful plutonium implosion weapon that exploded with a force equal to 20 kilotons of TNT.
Nuclear Reactor
– It has been possible to control the fission of U-235 so that energy is released slowly at a usable rate.
– Controlled fission is carried out in a specially designed plant called a nuclear power reactor or simply a nuclear reactor.
– The chief components of a nuclear reactor are:
(1) U-235 fuel rods which constitute the (fuel core).
– The fission of U-235 produces heat energy and neutrons that start the chain reaction.
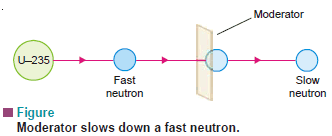
(2) Moderator which slows down or moderates the neutrons.
– The most commonly used moderator is ordinary water.
– Graphite rods are sometimes used.
– Neutrons slow down by losing energy due to collisions with atoms/molecules of the moderator.
(3) Control rods which control the rate of fission of U-235.
– These are made of boron-10 or cadmium, which absorbs some of the slowed neutrons.
– Thus the chain reaction is prevented from going too fast.
(4) Coolant which cools the fuel core by removing heat produced by fission.
– Water used in the reactor serves both as a moderator and coolant.
– Heavy water (D2O) is even more efficient than light water.
(5) Concrete shield which protects the operating personnel and environments from destruction in case of leakage of radiation
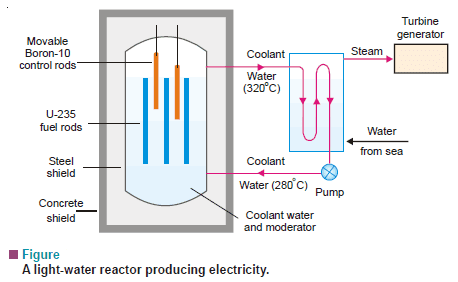
Light-water Nuclear power plant
– Most commercial power plants today are ‘light-water reactors’.
– In this type of reactor, U 235 fuel rods are submerged in water.
– Here, water acts as a coolant and moderator.
– The control rods of boron-10 are inserted or removed automatically from spaces in between the fuel rods.
– The heat emitted by the fission of U-235 in the fuel core is absorbed by the coolant.
– The heated coolant (water at 300°C) then goes to the exchanger.
– Here the coolant transfers heat to sea water which is converted into steam.
– The steam then turns the turbines, generating electricity.
– A reactor once started can continue to function and supply power for generations.
– About 15 percent of consumable electricity in the U.S.A. today is provided by light water reactors.
– India’s first nuclear plant went into operation in 1960 at Tarapur near Mumbai.
– Another plant has been set up at Narora in Uttar Pradesh.
– While such nuclear power plants will be a boon for our country, they could pose a serious danger to environment.
– In May 1986, the leakage of radioactive material from the Chernobyl nuclear plant in USSR played havoc with life and property around.
– Disposal of reactor waste poses another hazard.
– The products of fission e.g., Ba-139 and Kr 92, are themselves radioactive.
– They emit dangerous radiation for several hundred years.
– The waste is packed in concrete barrels which are buried deep in the earth or dumped in the sea.
– However, the fear is that any leakage and corrosion of the storage vessels may eventually contaminate the water supplies.
Breeder Reactor
– We have seen that uranium-235 is used as a reactor fuel for producing electricity.
– But our limited supplies of uranium-235 are predicted to last only for another fifty years.
– However, nonfissionable uranium-238 is about 100 times more plentiful in nature.
– This is used as a source of energy in the so-called breeder reactors which can supply energy to the world for 5,000 years or more.
– Here the uranium-235 core is covered with a layer or ‘blanket’ of uranium-238.
– The neutrons released by the core are absorbed by the blanket of uranium-238.
– This is then converted to fissionable plutonium-239.
– It undergoes a chain reaction, producing more neutrons and energy.
– The above reaction sequence produces three neutrons and consumes only two.
– The excess neutron goes to convert more uranium to plutonium-239.
– Thus the reactor produces or ‘breeds’ its fuel and hence its name.
– Several breeder reactors are now functioning in Europe.
– However, there is opposition to these reactors because the plutonium so obtained can be used in the dreaded H-bomb.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.