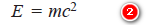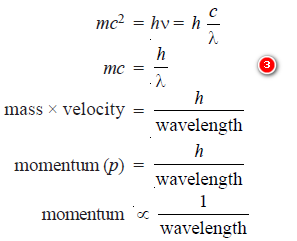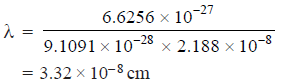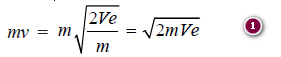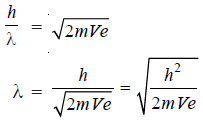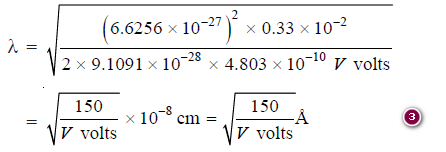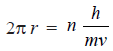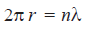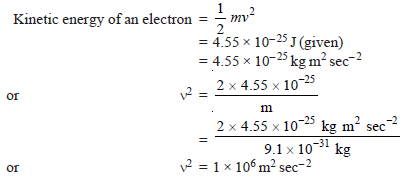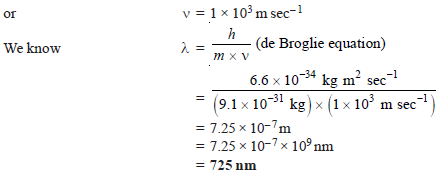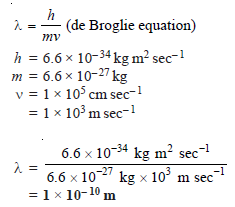De Broglie Equation – The Wave Nature of Electron
De Broglie Equation
– de Broglie had arrived at his hypothesis (de Broglie equation) with the help of Planck’s Quantum Theory and Einstein’s Theory of Relativity.
– He derived a relationship between the magnitude of the wavelength associated with the mass (m) of a moving body and its velocity.
– According to Planck, the photon energy (E) is given by the equation:
(h): Planck’s constant
(v): the frequency of radiation.
– By applying Einstein’s mass energy relationship, the energy associated with photon of mass (m) is given as
where (c) is the velocity of radiation
– Comparing equations (1) and (2):
– The equation (3) is called de Broglie equation and may be put in words as The momentum of a particle in motion is inversely proportional to wavelength, Planck’s constant (h) being the constant of proportionality.
– The wavelength of waves associated with a moving material particle (matter waves) is called de Broglie’s wavelength.
– The de Broglie equation is true for all particles, but it is only with very small particles, such as electrons, that the wave-like aspect is of any significance.
– Large particles in motion though possess wavelength, but it is not measurable or observable.
– Let us, for instance, consider de Broglie’s wavelengths associated with two bodies and compare their values:
(a) De Broglie Equation For a large mass
– Let us consider a stone of mass 100 g moving with a velocity of 1000 cm/sec.
– The de Broglie’s wavelength λ will be given as follows :
– This is too small to be measurable by any instrument and hence no significance.
(b) De Broglie Equation For a small mass
– Let us now consider an electron in a hydrogen atom.
– It has a mass = 9.1091 × 10–28 g and moves with a velocity 2.188 × 10–8cm/sec.
– The de Broglie’s wavelength (λ) is given as:
– This value is quite comparable to the wavelength of X-rays and hence detectable.
– It is, therefore, reasonable to expect from the above discussion that:
– Everything in nature possesses both the properties of particles (or discrete units) and also the properties of waves (or continuity).
– The properties of large objects are best described by considering the particulate aspect while properties of waves are utilized in describing the essential characteristics of extremely small objects beyond the realm of our perception, such as electrons.
The Wave Nature of Electron
– de Broglie revolutionary suggestion that moving electrons had waves of definite wavelength associated with them, was put to the acid test by Davison and Germer (1927).
– They demonstrated the physical reality of the wave nature of electrons by showing that a beam of electrons could also be diffracted by crystals just like light or X-rays.
– They observed that the diffraction patterns thus obtained were just similar to those in case of X-rays.
– It was possible that electrons by their passage through crystals may produce secondary X-rays, which would show diffraction effects on the screen.
– Thomson ruled out this possibility, showing that the electron beam as it emerged from the crystals, underwent deflection in the electric field towards the positively charged plate.
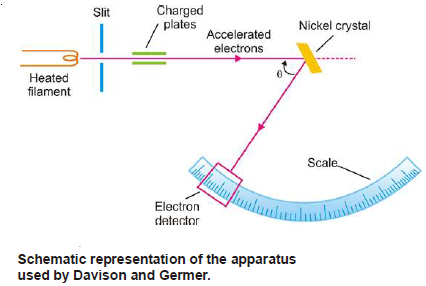
Davison and Germers Experiment
– In their actual experiment, Davison and Germer studied the scattering of slow moving electrons by reflection from the surface of nickel crystal.
– They obtained electrons from a heated filament and passed the stream of electrons through charged plates kept at a potential difference of (V) esu.
– Due to the electric field of strength (V × e) acting on the electron of charge (e), the electrons emerge out with a uniform velocity (v) units.
– The kinetic energy ½ mv2 acquired by an electron due to the electric field shall be equal to the electrical force.
– Thus,
– Multiplying by m on both sides,
– But according to de Broglie’s relationship

– Comparing (1) and (2):
– Substituting for
- h = 6.6256 × 10–27erg-sec,
- m = 9.1091 × 10–28 g,
- e = 4.803 × 10–10 esu,
– Changing V esu to V volts by using the conversion factor ⅓× 10−2, we have:
– If a potential difference of 150 volts be applied, the wavelength of electrons emerging out is λ = 1 Å.
– Similarly, if a potential difference of 1500 volts be created, the electrons coming out shall have a wavelength 0.1 Å.
– It is clear, therefore, that electrons of different wavelengths can be obtained by changing the potential drop.
– These wavelengths are comparable with those of X-rays and can undergo diffraction.
Apparatus used by Davison and Germer
– The electrons when they fall upon the nickel crystal, get diffracted.
– Electrons of a definite wavelength get diffracted along definite directions.
– The electron detector measures the angle of diffraction (say θ) on the graduated circular scale.
– According to Bragg’s diffraction equation, the wavelength λ of the diffracted radiation is given by ( λ = d sin θ), where d is a constant (= 2.15 for Ni crystal) and θ the angle of diffraction.
– By substituting the experimental value of (θ) in Bragg’s equation (λ = d sin θ), the wavelength of electrons may be determined.
– This wavelength would be found to agree with the value of (λ), as obtained from equation (3).
– Since diffraction is a property exclusively of wave motion, Davison and Germer’s (electron diffraction) experiment established beyond doubt the wave nature of electrons.
– We have described earlier that electrons behave like particles and cause mechanical motion in a paddle wheel placed in their path in the discharge tube.
– This proves, therefore, that electrons not only behave like (particles) in motion but also have ‘wave properties’ associated with them.
– It is not easy at this stage to obtain a pictorial idea of this new conception of the motion of an electron.
– But the application of de Broglie equation to Bohr’s theory produces an important result.
De Broglie equation accommodated in Bohr’s orbit
– The quantum restriction of Bohr’s theory for an electron in motion in the circular orbit is that the angular momentum (mvr) is an integral multiple (n) of (h/2π).
– That is,

– On rearranging, we get:
– Putting the value of (h/mv) from equation (1), we have:
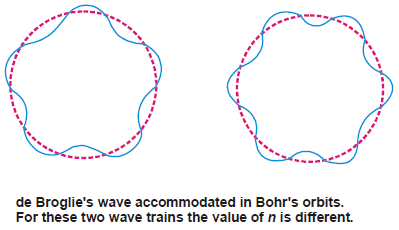
– Now the electron wave of wavelength (λ) can be accommodated in Bohr’s orbit only if the circumference of the orbit, (2πr), is an integral multiple of its wavelength.
– Thus de Broglie’s idea of standing electron waves stands vindicated. However, if the circumference is bigger, or smaller than (nλ), the wave train will go out of phase and the destructive interference of waves causes radiation of energy.
Solved Problem on De Broglie Equation
Problem (1): Calculate the wavelength of an electron having kinetic energy equal to 4.55 × 10–25 J. (h = 6.6 × 10–34 kg m2sec–1 and mass of electron = 9.1 × 10– 31 kg).
Solution:
Problem (2): Calculate the wavelength of an α particle having mass 6.6 × 10–27 kg moving with a speed of 105 cm sec– 1(h = 6.6 × 10–34 kg m2 sec–1).
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.