Electronegativity and Electron Affinity
– In this subject, we will discuss the difference Between Electronegativity and Electron Affinity
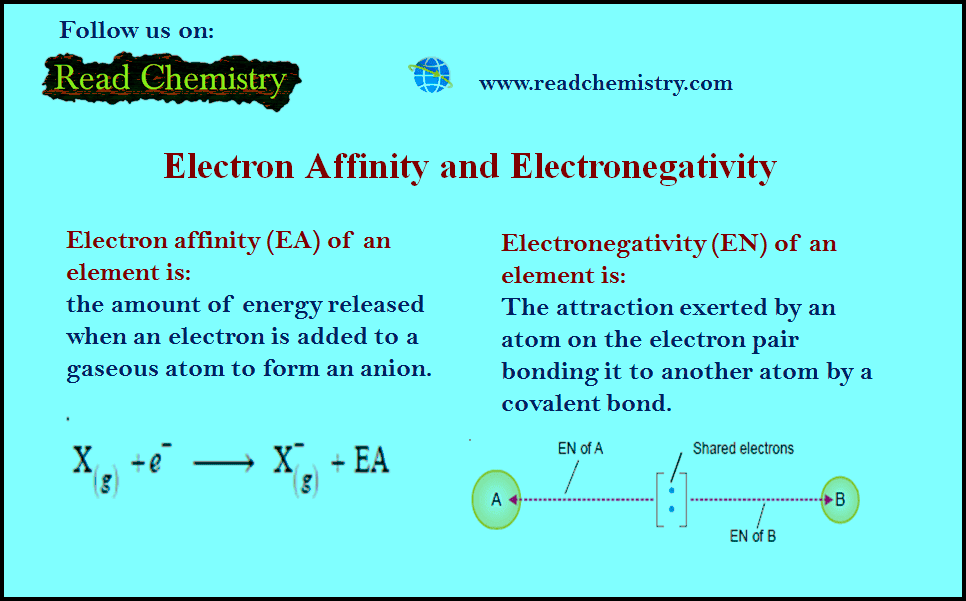
Electron Affinity
– A neutral atom can accept an electron to form a negative ion. In this process, in general, energy is released.
– The electron affinity (EA) of an element is the amount of energy released when an electron is added to a gaseous atom to form an anion.
– The energy involved in the addition of the first electron is called first-electron affinity; the energy involved in the addition of a second electron is called second-electron affinity; and so on. Thus:
– The electron affinity of an element measures the ease with which it forms an anion in the gas phase.
– Electron affinities are difficult to measure and accurate values are not known for all elements.
– They are expressed in kJ mol–1.
Trends in Electron Affinities
– The factors that determine the magnitude and sign of electron affinities are similar to those used to explain the ionization energies of elements.
– In fact, the electron affinity of a neutral atom may be thought of simply as equivalent to the ionization energy of the singly charged negative ion of the atom.

– The first-electron affinities of elements in the Periodic table are expected to show trends analogous to those of ionization energies.
(a) Increase across a Period
– The values of electron affinities for Period (2) are listed below:
– As we proceed from left to right, the general trend is the increase of electron affinities. Be, N and Ne are exceptions.
– Elements having relatively stable electronic configurations find it difficult to accept an electron readily.
– The atom of Be has the configuration 1s2 2s2.
– The 1s subshell is completely filled and, therefore, the electron being added must go to a subshell of considerably higher energy.
– This gives rise to negative electron affinity for Be.
– The atom of N (1s22s2 , 2px1 ,2py1 ,2pz1) has half-filled 2p subshells, a condition of extra stability.
– Therefore the electron affinity of N would be less than expected.
– The electron affinity of Neon is low because it has a stable outer-shell octet. Its atom shows little tendency to start a new shell.
(b) Decrease down a Group
– The values of electron affinities for halogens (Group VII) are given below.
– The electron affinities show a general decrease from top to bottom.
– This is so because the valence shell is progressively farther from the nucleus.
– The value for fluorine, however, is out of line as it has a smaller atomic size than that of chlorine.
(c) Second electron affinity negative
– The second electron affinity of an element is always negative.
– This is on account of repulsion between the electron being added and the already negatively charged atom.
– For example,
Electronegativity
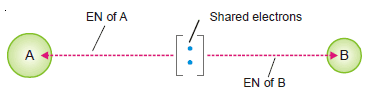
– In a molecule A – B the electrons forming the covalent bond are attracted by atom A as well as by B.
– This attraction is measured in terms of what we call electronegativity, EN.
– Electronegativity is The attraction exerted by an atom on the electron pair bonding it to another atom by a covalent bond.
– It is evident that an atom of high electronegativity will attract the shared electron pair away from one of lower electronegativity.
– Thus the former atom will acquire a partial negative charge while the other atom will get a partial positive charge.
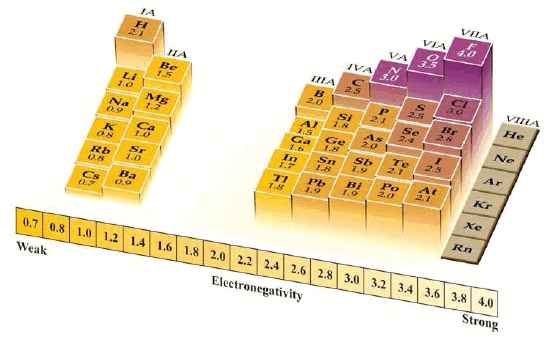
Electronegativity Values
– Using measured values of bond energies, Pauling devised a set of electronegativity values.
– He allotted a value of 4 to the most electronegative atom, namely fluorine, and assigned values to the atoms of other elements.
Trend in Electronegativity
– The variations in electronegativities of elements in the Periodic table are similar to those of ionization energies and electron affinities.
(1) Increase across a Period
– The values of electronegativities increase as we pass from left to right in a Period. Thus for Period (2) we have:
– This is so because the attraction of bonding electrons by an atom increases with increase of nuclear charge (At. No.) and decrease of atomic radius.
– Both these factors operate as we move to the right in a Period.
(2) Decrease down a Group
– The electronegativities of elements decrease from top to bottom in a Group.
– Thus for Group VII
– The decrease trend is explained by more shielding electrons and larger atomic radius as we travel down a Group.
Importance of Electronegativity
– The electronegativities of elements are widely used throughout the study of Chemistry.
– Their usefulness will be discussed at appropriate places.
– The important applications of electronegativities are listed below:
(1) In predicting the polarity of a particular bond.
– The polarity of a bond, in turn, shows the way how the bond would break when attacked by an organic reagent.
(2) In predicting the degree of ionic character of a covalent bond.
(3) In predicting of inductive effects in organic chemistry.
(4) In understanding the shapes of molecules.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.