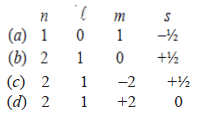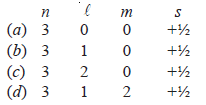MCQ on Chapter: structure of atom – wave mechanical approach
MCQ on the structure of the atom
– In this subject, you will find 50 questions and answers MCQ on Chapter structure of atom – wave mechanical approach
1. The number of unpaired electrons in oxygen atom is_______
(a) 1
(b) 2
(c) 3
(d) 4
Answer. (b)
2. The wavelength of large objects is of no significance as it is too _______ to be measurable.
(a) small
(b) large
(c) heavy
(d) none of these
Answer. (a)
3. de Broglie equation is_______
(a) λ = h/m v
(b) λ = m v/h
(c) λ = h m v
(d) λ = hν / m
Answer. (a)
4. “It is impossible to determine simultaneously the position and velocity with accuracy of a small particle like electron”. This statement is_______
(a) Heisenberg’s uncertainty principle
(b) de Broglie principle
(c) Planck’s law
(d) Aufbau’s principle
Answer. (a)
5. The relation Δx × Δp =h /4π represents_______
(a) de Broglie equation
(b) Heisenberg’s uncertainty principle
(c) Schrödinger’s wave equation
(d) Pauli’s exclusion principle
Answer. (b)
6. In Schrödinger’s wave equation, the symbol ψ represents the_______
(a) wavelength of the spherical wave
(b) amplitude of the spherical wave
(c) frequency of the spherical wave
(d) none of these
Answer. (b)
7. The energy of electron in an atomic orbital is always _______.
(a) different
(b) zero
(c) infinite
(d) same
Answer. (d)
8. An orbital is the space around the nucleus where the probability of finding electron is_______
(a) always zero
(b) maximum
(c) minimum
(d) always infinite
Answer. (b)
9. The Principal quantum number ‘n’ represents_______
(a) average size of the electron cloud
(b) average energy of the electron
(c) average distance of the electron from the nucleus
(d) all of the above
Answer. (d)
10. The Principal quantum number is related to the_______
(a) orbital angular momentum
(b) size and shape of the orbital
(c) orientation of the orbital
(d) average size of the orbital
Answer. (d)
11. The quantum number that defines the shape of the orbital occupied by the electron is_______
(a) principal quantum number
(b) azimuthal quantum number
(c) magnetic quantum number
(d) spin quantum number
Answer. (b)
12. The angular momentum of the electron is defined by the quantum number that is denoted as_______
(a) n
(b) l
(c) m
(d) s
Answer. (b)
13. The total number of sublevels in each principal level is equal to_______
(a) spin quantum number
(b) magnetic quantum number
(c) azimuthal quantum number
(d) principal quantum number
Answer. (d)
14. The quantum number which accounts for the splitting up of spectral lines (Zeeman effect) is_______
(a) principal quantum number
(b) azimuthal quantum number
(c) magnetic quantum number
(d) spin quantum number
Answer. (c)
15. For a given value of principal quantum number the order of increasing energy for different subshells is_______
(a) s < p < d < f
(b) p < d < f < s
(c) d < f < p < s
(d) f < d < p < s
Answer. (a)
16. The px, py and pz orbitals are called degenerate orbitals as they have_______
(a) equal energy
(b) same orientation in space
(c) same size
(d) none of these
Answer. (a)
17. A nodal plane separates the two lobes of a p-orbital. There is _______ likelihood of finding the electron on this plane.
(a) no
(b) every
(c) either of these
(d) none of these
Answer. (a)
18. The total values of magnetic quantum number for a given value of azimuthal quantum number is_______
(a) 2l
(b) 2l + 1
(c) 2l –1
(d) 2l – 2
Answer. (b)
19. “No two electrons in an atom can have same set of four identical quantum numbers”. It is the statement of_______
(a) Aufbau principle
(b) Hund’s rule
(c) Pauli’s exclusion principle
(d) none of these
Answer. (c)
20. The orbital with n = 3 and l = 2 is_______
(a) 3s
(b) 3p
(c) 3d
(d) 3f
Answer. (c)
21. 4s orbital has lesser energy than 3d orbital because it has_______
(a) greater value of n
(b) lesser value of l
(c) lesser value of n + l
(d) l = 0
Answer. (c)
22. The maximum number of electrons that can be accommodated in f-subshell is_______
(a) 5
(b) 7
(c) 10
(d) 14
Answer. (d)
23. The energy associated with electrons in s, p, d, and f orbitals of a particular principal quantum number in hydrogen atom is in the order_______
(a) s = p = d = f
(b) s < p < d < f
(c) p < d < f < s
(d) f < d < p < s
Answer. (a)
24. For a multi-electron atom, the energy associated with electrons is s, p, d and f orbitals of a particular quantum number is in the order_______
(a) s = p = d = f
(b) s < p < d < f
(c) p < d < f < s
(d) d < f < s < p
Answer. (b)
25. The two electrons in the first shell will differ in the values for_______
(a) n
(b) l
(c) m
(d) s
Answer. (d)
26. Which one of the following sets of quantum numbers is not allowed?
Answer. (d)
27. Which of the following is incorrect for 3d orbital?
Answer. (d)
28. The value of azimuthal quantum number for last electron of N-atom is_______
(a) 0
(b) 1
(c) 2
(d) 3
Answer. (b)
29. The maximum number of electrons in a subshell is given by the equation
(a) n2
(b) 2n2
(c) 2l –1
(d) 2l +1
Answer. (d)
30. Out of the following, which is the correct set of quantum numbers for the outermost electron of potassium atom (Z = 19)?
Answer. (d)
31. According to de Broglie’s equation, the momentum of a particle in motion is _______ proportional to wavelength.
(a) inversely
(b) directly
(c) is not
(d) none of these
Answer. (a)
32. The number of unpaired electrons in chromium atom (Z = 24) is_______
(a) 1
(b) 2
(c) 3
(d) 6
Answer. (d)
33. In nitrogen atom there are three unpaired electrons. These are having _______ direction of spin.
(a) same
(b) different
(c) similar
(d) none of these
Answer. (a)
34. The maximum number of electrons that can be accommodated in s, p, d and f orbitals is_______
(a) 1, 2, 3 and 4 respectively
(b) 1, 2, 4 and 8 respectively
(c) 2, 4, 6 and 8 respectively
(d) 2, 6, 10 and 14 respectively
Answer. (d)
35. The sum of all quantum numbers of the electron of hydrogen atom is_______
(a) –1/2
(b) 1
(c) 3/2
(d) +1/2
Answer. (c)
36. The sum of all quantum numbers of the last electron in lithium atom is_______
(a) 3/2
(b) 2
(c) 5/2
(d) 3
Answer. (c)
37. The value of azimuthal quantum number for the electrons present in 5s-orbital is_______
(a) 0
(b) 1
(c) 2
(d) 5
Answer. (a)
38. According to Pauli’s exclusion principle two electrons can occupy the same orbital only if they have_______ direction of spin.
(a) different
(b) same
(c) similar
(d) none of these
Answer. (a)
39. In the ground state of an atom, the electrons tend to occupy the available orbitals in the _______ order of energies.
(a) increasing
(b) decreasing
(c) any
(d) none of these
Answer. (a)
40. Amongst 3d, 4s and 4p orbitals, the order of increasing energies is_______
(a) 3d < 4p < 4s
(b) 4s < 4p < 3d
(c) 4p < 4s < 3d
(d) 3d < 4s < 4p
Answer.. (d)
41. While comparing the energies of two orbitals we compare their (n + l ) values the orbital with _______ (n + l ) value will have _______ energy.
(a) lower, lower
(b) higher, lower
(c) lower, higher
(d) none of these
Answer. (a)
42. When two orbitals have the same (n + l ) value, the orbital with lower value of _______ has lower energy.
(a) principal quantum number
(b) azimuthal quantum number
(c) magnetic quantum number
(d) spin quantum number
Answer. (a)
43. After filling the 4p-orbitals, an electron will enter in_______
(a) 4d
(b) 4f
(c) 5s
(d) 3d
Answer. (c)
44. If the electronic configuration of nitrogen (at no = 7) is written as 1s2 2s2 2px2 2py1, it would violate_______
(a) Aufbau principle
(b) Pauli’s exclusion principle
(c) Hund’s rule of maximum multiplicity
(d) none of these
Answer. (c)
45. The outermost electronic configuration of manganese (at. no. = 25) is_______
(a) 3d5 4s2
(b) 3d6 4s1
(c) 3d7 4s0
(d) 3d6 4s2
Answer. (a)
46. The subshell, which does not exist, has the quantum numbers_______
(a) n = 2 , l = 0
(b) n = 2 , l = 1
(c) n = 2 , l = 2
(d) n = 3 , l = 0
Answer. (c)
47. The ground state electronic configuration of carbon atom has _______ pairs and _______ unpaired electrons
(a) 2, 2
(b) 1, 2
(c) 2, 1
(d) 2, 3
Answer. (a)
48. Two electrons occupying the same orbital have different _______.
(a) principal quantum number
(b) azimuthal quantum number
(c) magnetic quantum number
(d) spin quantum number
Answer. (d)
49. If the value of azimuthal quantum number is 2, there will be _______ values for magnetic quantum number.
(a) 2
(b) 3
(c) 4
(d) 5
Answer. (d)
50. The energy needed to remove a single electron (most loosely bound) from an isolated gaseous atom is called_______
(a) ionisation energy
(b) electron affinity
(c) kinetic energy
(d) electronegativity
Answer. (a)
51. Generally speaking, the ionisation energies increase when we move_______
(a) from left to right in the periodic table
(b) from top to bottom in a group
(c) from right to left in the periodic table
(d) none of these
Answer. (a)
52. The ionisation energy of Boron (Z = 5) is lesser than that of Beryllium (Z = 4). It is because_______
(a) Be has an incomplete 2s orbital
(b) Be has two pairs of electrons
(c) 2p orbital is already higher in energy than 2s orbital
(d) none of the above
Answer. (c)
53. A neutral atom can accept an electron to form an anion. This process involves_______
(a) loss of energy
(b) gain of energy
(c) no change in energy
(d) none of these
Answer. (a)
54. Electron affinity is expressed in_______
(a) g mol–1
(b) kJ mol–1
(c) cal g–1
(d) kJ g–1
Answer. (b)
55. When we move from left to right across a period, the electron affinity in general
(a) remains the same
(b) decreases
(c) increases
(d) becomes zero
Answer. (c)
56. The attraction exerted by an atom on the electron pair bonding it to another atom by covalent bond is called_______
(a) ionisation energy
(b) electron affinity
(c) electronegativity
(d) none of these
Answer. (c)
57. The most electronegative element in the periodic table is_______
(a) ceasium
(b) chlorine
(c) fluorine
(d) barium
Answer. (c)
58. The values of electronegativities _______ as we move from left to right in a period.
(a) increase
(b) decrease
(c) remain the same
(d) none of these
Answer. (a)
59. The electron affinities _______ from top to bottom in a group
(a) increase
(b) decrease
(c) remain the same
(d) none of these
Answer. (b)
60. The second electron affinity of an element is always_______
(a) zero
(b) positive
(c) negative
(d) infinity
Answer. (c)
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.