Different Types of Heat of Reaction (Enthalpy)
– The heat of reaction or enthalpy changes accompanying chemical reactions are expressed in different ways, depending on the nature of the reaction. These are discussed below.
(1) Heat of Formation
– The heat of formation of a compound is defined as The change in enthalpy that takes place when one mole of the compound is formed from its elements.
– It is denoted by ΔHf.
– For example, the heat of formation of ferrous sulfide and acetylene may be expressed as:
– Similarly, the reaction between gaseous hydrogen and gaseous chlorine to form gaseous hydrogen chloride is represented by the equation:
– It may be noted in this case that – 44.0 kcal is not the heat of formation of hydrogen chloride because this amount of heat is evolved when two moles of hydrogen chloride are formed.
– The heat of formation of hydrogen chloride, therefore, would be – 44.0/2 = – 22.0 kcal and the equation can be written as:
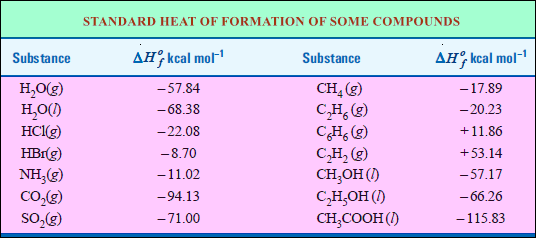
Standard Heat of Formation
– The standard heat of formation of a compound is defined as The change in enthalpy that takes place when one mole of a compound is formed from its elements, all substances being in their standard states (298 K and 1 atm pressure).
– The standard heat of formation of some compounds is given in the following table:
– By convention, the standard heat of formation of all elements is assumed to be zero.
Standard Heat of Reaction (ΔHº) from Standard Heat of Formation (ΔHof)
– We can calculate the heat of reaction under standard conditions from the values of standard heat of formation of various reactants and products.
– The standard heat of reaction is equal to the standard heat of formation of products minus the standard heat of formation of reactants.
– Let us consider a general reaction:
aA + bB → cC + dD
– The standard heat of reaction is given by:
Solved Problem
Problem (1): Calculate ΔHº for the reaction:
CO2(g) + H2(g) → CO(g) + H2O(g)
given that ΔHof for CO2(g), CO(g) and H2O(g) are – 393.5, – 111.31 and – 241.80 kJ mol–1 respectively.
Solution
Here we have:
Problem (2): The standard heats of formation of C2H5OH(l), CO2(g), and H2O(l) are – 277.0, –393.5, and –285.5 kJ mol–1 respectively. Calculate the standard heat change for the reaction:
Solution:
Here we have:
(2) Heat of Combustion
– The heat of combustion of a substance is defined as The change in enthalpy of a system when one mole of the substance is completely burnt in excess of air or oxygen.
– It is denoted by ΔHc.
– For example, the heat of combustion of methane is – 21.0 kcal (= 87.78 kJ) as shown by the equation:
Now consider the chemical equations:
– It may be noted that – 94.3 kcal and not – 26.0 kcal is the heat of combustion of carbon as the combustion is complete only in the first reaction.
– In the second case, oxidation has converted carbon to carbon monoxide and is by no means complete as carbon monoxide can be further oxidized to carbon dioxide.
– It should be noted clearly that the heat of combustion of a substance (ΔHc) is always negative.
– Heat energy is evolved during the process of combustion i.e., ΔHc = – ve.
Applications of The Heat of Combustion
(1) Calculation of heat of formation.
– Since the heats of combustion of organic compounds can be determined with considerable ease, these are employed to calculate their heats of formation.
– The direct determination of these is often impossible.
(2) Calorific value of foods and fuels.
– The calorific value is defined as the amount of heat produced in calories (or joules) when one gram of a substance is completely burnt.
– It is expressed in cal g–1 or kcal g–1 or kJ g–1.
– Let us compare the calorific values of methane and ethane.
– Their heats of combustion are – 890.3 kJ and – 1559.7 kJ.
– These combustion reactions are expressed as:
– In the case of methane heat produced per gram is 890.3/16 = 55.64 kJ g–1while for ethane it is –1559.7/30 = 51.90 kJ g–1.
– Thus methane has better fuel efficiency than ethane as it produces more heat per gram.
(3) Deciding constitution.
– Heat of combustion of organic compounds is to a large extent an additive property, as shown by the fact that in a homologous series, the difference between the heats of combustion of successive members is nearly constant and is equal to 158 Cals.
– Constants corresponding to the heats of combustion of various atoms and linkages have been worked out.
– The heat of combustion of an organic substance can be calculated from its probable structural formula by adding up the values of the constants corresponding to the atoms and linkages involved therein.
– If the value so obtained comes out to be the same as the experimental value of the heat of combustion of the compound, the assumed formula must be correct.
– In this way, Kekule’s formula for benzene with alternate double and single linkages has been supported as the calculated value of the heat of combustion of benzene according to this formula agrees with the actual heat of combustion.
– The heat of combustion of organic compounds has thus proved very valuable in deciding their chemical constitution.
Solved Problem
Calculate the standard heat of formation of propane (C3H8) if its heat of combustion is – 2220.2 kJ mol–1. The heats of formation of CO2(g) and H2O(l) are – 393.5 and – 285.8 kJ mol–1 respectively.
Solution
We are given:
(3) Heat of Solution
– Heat changes are usually observed when a substance is dissolved in a solvent.
– When a reaction takes place in solution, the heat of solution of reactants and products must be taken into consideration.
– The heat of solution is defined as the change in enthalpy when one mole of a substance is dissolved in a specified quantity of solvent at a given temperature.
– For example, when one mole of copper sulfate is dissolved in water so that we get one molar solution, the heat absorbed is 78.5 kJ.
– If the solution so obtained is further diluted, there will again be a change in enthalpy.
– If we go on diluting the solution, a stage will come when further dilution produces no thermal effect.
– This state is called the state of infinite dilution.
– To avoid the quantity of the solvent, we have to incorporate the idea of infinite dilution in our definition which may be stated as:
– The heat of solution is the change in enthalpy when one mole of a substance is dissolved in a solvent so that further dilution does not give any change in enthalpy.
– The heat of solution can also be expressed as:
– Heat of solution of an electrolyte may be due to energy change involved during ionization or some hydrate formation as in the case of sulphuric acid.
– Usually, heat is absorbed when ions are torn apart from each other in the process of solution, and heat is evolved during hydrate formation.
– With a salt as sodium chloride the heat of separation of ions just equals the heat of hydration and there is very little heat effect.
(4) Heat of Neutralization
– The heat of neutralization is defined as the change in heat content (enthalpy) of the system when one gram equivalent of an acid is neutralized by one gram equivalent of a base or vice versa in a dilute solution.
– The following may be considered as typical examples of the heat of neutralization:
– It may be concluded from the above data that the heat of neutralization of a strong acid and strong base is –13.7 kcal, no matter which acid or base is employed.
– This regularity has been explained satisfactorily with the help of the theory of ionization.
– If HA and BOH represent any strong acid and any strong base respectively and equivalent amounts of these in dilute solution are mixed, we have:
– Disregarding the ions which are present on both sides of the equation, we get:
– Thus the heat of neutralization of an acid and a base is merely the heat of formation of water from hydrogen and hydroxyl ions.
– When weak acids or weak bases are neutralized by strong bases or strong acids respectively, the heat of neutralization differs widely from – 13.7 kcal.
– This is shown by the following examples:
– In such cases, the neutralization process involves not only the union of hydrogen and hydroxyl ions but also the dissociation of the weak acid or base.
How to calculate The heat of neutralization
– The measured heat of neutralization is, therefore, equal to the heat given out in the union of H+ (aq) and OH– (aq) ions plus the heat accompanying the dissociation of weak acid or weak base.
– The neutralization of NH4OH with HCl, for example, can be represented as:
– But the measured heat of neutralization is – 12.3 kcals.
– Therefore,
– Hence the heat of dissociation of NH4OH is 1.4 kcal i.e., 1.4 kcal of heat is absorbed when one mole of ammonium hydroxide is dissociated into ions.
– In general, the heat of dissociation of a weak acid or weak base may be defined as the change in enthalpy of the system when one mole of it is dissociated into ions.
Energy Changes During Transitions or Phase Changes
– The three states of matter – solid, liquid, and gas differ from one another in the arrangement of their constituent particles.
– The magnitudes of intermolecular forces acting between the particles in these states are also different.
– It is a common observation that when a solid is converted into the liquid state, energy is to be supplied.
– This energy is spent in breaking the intermolecular forces in the solid which are of high magnitude.
– Whenever there is a change in the state of matter (solid → liquid or liquid → gas), the process is called phase change or transition.
– It is also accompanied by the change in enthalpy or heat content of the system.
(5) Heat of Fusion
– Heat of Fusion is defined as the heat change (or enthalpy change) when one mole of a solid substance is converted into the liquid state at its melting point.
– As an example, we can take the melting of one mole of ice at its melting point, 0ºC or 273 K.
– The process can be represented as:
and is accompanied by the absorption of 1.43 kcal of heat.
– From the values of fusion of various substances we can compare their magnitudes of intermolecular forces.
– The greater the heat of fusion of a substance higher the magnitude of intermolecular forces.
(6) Heat of Vaporization
– The heat of vaporization is defined as the heat change (or enthalpy change) when one mole of liquid is converted into vapor or gaseous state at its boiling point.
– For example, when one mole of water is converted into steam at 100ºC or 373 K, the heat absorbed is 9.71 kcal which is the heat of vaporization of water.
– The change can be represented as:
– The heats of vaporization of ethyl alcohol (C2H5OH) and benzene (C6H6) are 7.29 kcal mol-1 and 7.36 kcal mol–1respectively.
– The values of heats of vaporization can also be used for the comparison of the magnitude of intermolecular forces of attraction in liquids.
(7) Heat of Sublimation
– Sublimation is a process when a solid changes directly into the gaseous state without changing into the liquid state.
– It occurs at a temperature below the melting point of the solid.
– Heat of sublimation is defined as the heat change (or enthalpy change) when one mole of a solid is directly converted into the gaseous state at a temperature below its melting point.
– For example, the heat of sublimation of iodine is 14.92 kcal mol–1.
– It can be represented as:
(8) Heat of Transition
– The heat of transition is defined as the change in enthalpy which occurs when one mole of an element changes from one allotropic form to another.
– For example, the transition of diamond into amorphous carbon may be represented as:
where – 0.016 kcal and – 1.028 kcal are heats of transition of monoclinic sulphur to rhombic sulphur and white phosphorus to red phosphorus respectively.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.

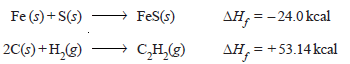
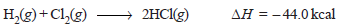
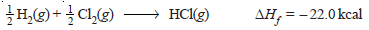
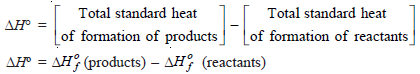

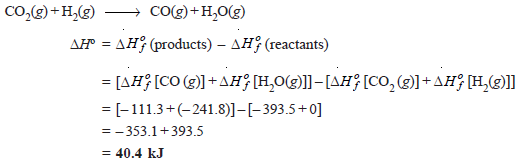
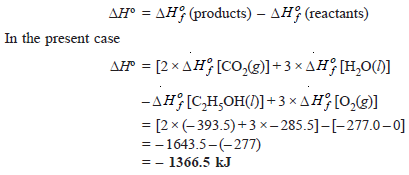
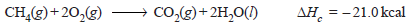

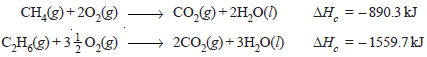
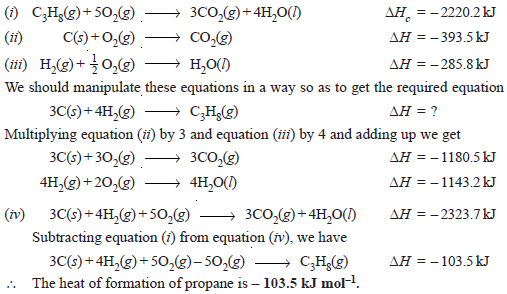
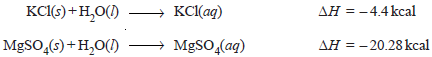
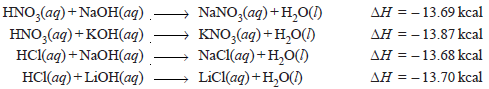
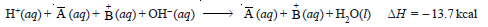

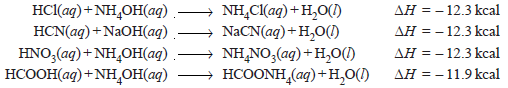
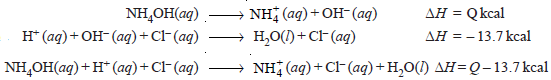
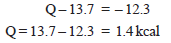



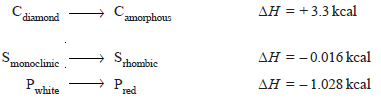



Well Precise