Enthalpy of Reaction
– For reactions involving solids and liquids only the change in volume (ΔV) is very small and the term P × ΔV is negligible. For such reactions, the Change of Enthalpy of Reaction ΔH is equal to ΔE.
Enthalpy of Reaction
– Thermochemical measurements are made either at (a) constant volume or (b) constant pressure.
– The magnitudes of changes observed under the two conditions are different.
– The change in internal energy (ΔE) is the heat change accompanying a chemical reaction at constant volume because no external work is performed.
– However at constant pressure not only does the change in internal energy take place but work is also involved because of expansion or contraction.
– In the laboratory, most of the chemical reactions are carried out at constant pressure (atmospheric pressure) rather than at constant volume.
– In order to study the heat changes for reactions taking place at constant pressure and constant temperature, chemists have introduced a new term called enthalpy.
Calculation of Enthalpy of system
– The enthalpy of a system is defined as the sum of the internal energy and the product of its pressure and volume. That is,
H = E + PV
where E is the internal energy, P is the pressure and V is the volume of the system. It is also called Heat content.
– Just like internal energy, enthalpy is also a function of the state and it is not possible to measure its absolute value. However, a change in enthalpy (ΔH) accompanying a process can be measured accurately and is given by the expression.
ΔH = Hproducts – Hreactants
= Hp– Hr
– Thus if ΔV be the change in volume in case of a reaction at constant temperature and pressure, the thermal effect observed will be the sum of the change in internal energy (ΔE) and the work done in expansion or contraction. That is,
ΔΗ = ΔE + P × ΔV
– Therefore, while the heat change in a process is equal to its change in internal energy ΔE at constant volume, it gives at constant pressure the enthalpy change ΔH. That is,
ΔE = Heat change in a reaction at constant volume
ΔH = Heat change in a reaction at constant pressure
– For reactions involving solids and liquids only the change in volume (ΔV) is very small and the term P × ΔV is negligible. For such reactions, ΔH is equal to ΔE.
– In the case of gases, however, we must specify whether the reaction has taken place at constant volume or at constant pressure because the value of P × ΔV is appreciable.
– Most of such reactions are, however, studied at constant pressure, and change in enthalpy (ΔH) is involved
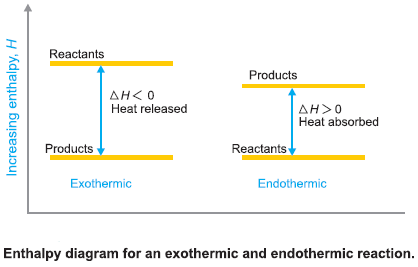
Exothermic and Endothermic Reactions
– Let us consider a general reaction at constant pressure,
A + B → C + D
– If HA, HB, HC and HD be the enthalpies of A, B, C and D respectively, the heat of reaction at constant pressure viz., ΔH is equal to the difference in enthalpies of the products and the reactants
i.e.,
ΔH = Hproducts – Hreactants
= (HC+ HD) – (HA + HB)
– The value of ΔH may be either zero, negative or positive.
– Where ΔH is zero, the enthalpies of the products and reactants being the same, the heat is evolved or absorbed.
– In case ΔH is negative, the sum of enthalpies of the products is less than that of the reactants and the difference in enthalpy is given out in the form of heat.
– Such reactions which are accompanied by the evolution of heat energy are called Exothermic reactions.
– When ΔH is positive, the enthalpy or heat content of the reactants and an equivalent of heat is absorbed by the system from the surroundings.
– Such reactions which are accompanied by absorption of heat are called Endothermic reactions.
– Thus for an exothermic reaction Hp < Hr and ΔH = – ve, for an endothermic reaction Hp > Hr and ΔH = +ve.
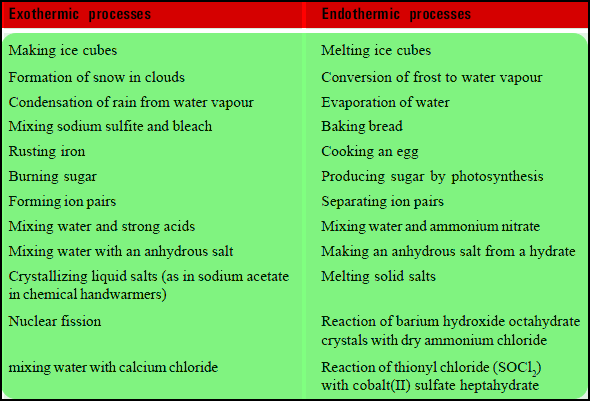
Examples of Exothermic and Endothermic Processes
– When trying to classify a process as exothermic or endothermic, watch how the temperature of the surroundings changes.
– An exothermic process releases heat and causes the temperature of the immediate surroundings to rise.
– An endothermic process absorbs heat and cools the surroundings.
Sign of ΔH and ΔE
– A negative sign of ΔH or ΔE shows that heat is evolved and the reaction is exothermic.
– A positive sign of ΔH or ΔE indicates that heat energy is absorbed and the reaction is endothermic.
Calculation of ΔH from ΔE and vice versa
– The enthalpy change of a reaction at constant pressure (ΔH) and internal energy change (ΔE) are related to each other as:
ΔH = ΔE + P × ΔV … (i)
– where ΔV is the change in volume due to expansion or contraction when measurement is done at constant pressure, P.
– Though heat changes of reactions are usually measured at constant pressure, it is sometimes necessary to carry out the reaction at constant volume as, for example, in the measurement of heat of combustion in a bomb calorimeter.
– The above relationship can be used, if desired, for the conversion of ΔH into ΔE and vice versa.
– Let us consider a reaction
aA + bB → cC +dD
– Change in the number of moles
= No. of moles of products – No. of moles of reactants
= (c + d) – (a + b) = Δn
– Let the volume occupied by one mole of the gas be V.
– Then, change in volume, ΔV = change in No. of moles × volume occupied by one mole of the gas.
ΔV = Δn × V
P × ΔV = P (Δn × V)
P × ΔV = PV × Δn … (ii)
– But
PV = RT (for one mole of gas)
– Putting RT in place of PV in equation (ii) we get:
PΔV = RT Δn
– Substituting the value of PΔV in equation (i) we get:
ΔH = ΔE + Δn RT
– It may be pointed out that while determining the value of ΔH, only the number of moles of gaseous reactants and products are taken into consideration.
– The value of gas constant R is taken either in calories or joules per degree per mol and is 1.987 cal (approximately 2 calories) or 8.314 joules.
Solved Problem on Enthalpy of Reaction
Problem (1): The heat of combustion of ethylene at 17ºC and at constant volume is – 332.19 kcals. Calculate the heat of combustion (Enthalpy of Reaction) at constant pressure considering water to be in liquid state. (R = 2 cal degree–1 mol–1)
Solution
– The chemical equation for the combustion of ethylene is:
Problem (2): The heat of combustion of carbon monoxide at constant volume and at 17ºC is – 283.3 kJ. Calculate its heat of combustion at constant pressure (R = 8.314 J degree–1 mol–1).
Solution
Problem (3): The heat of formation of methane at 298 K at constant pressure is – 17.890 kcal. Calculate its heat of formation at constant volume. (R = 1.987 cal degree–1 mol–1)
Solution
The thermochemical equation for the heat of formation of methane at 298 K at constant pressure is:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition.


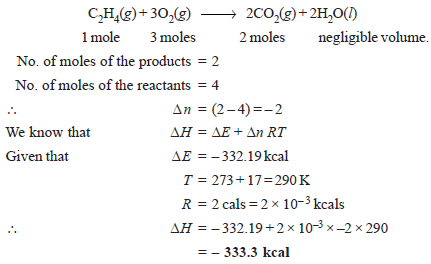

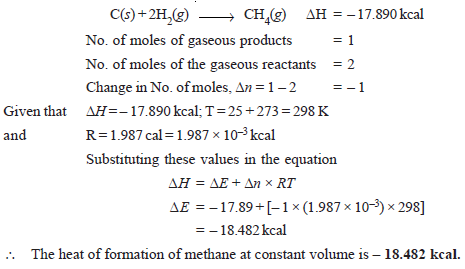


it is excellent