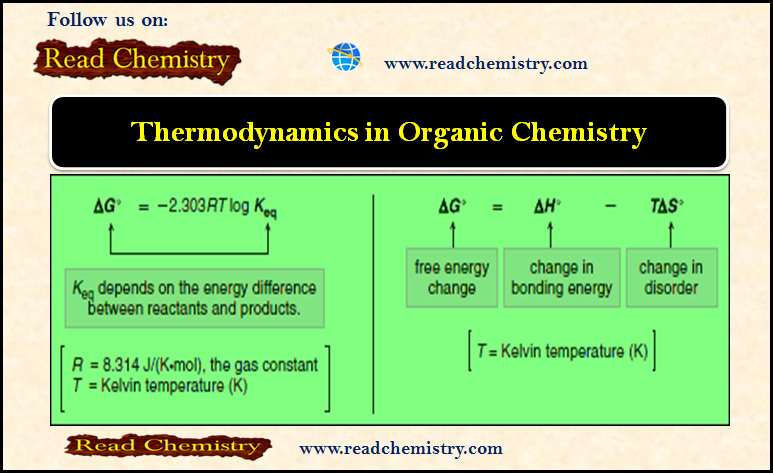
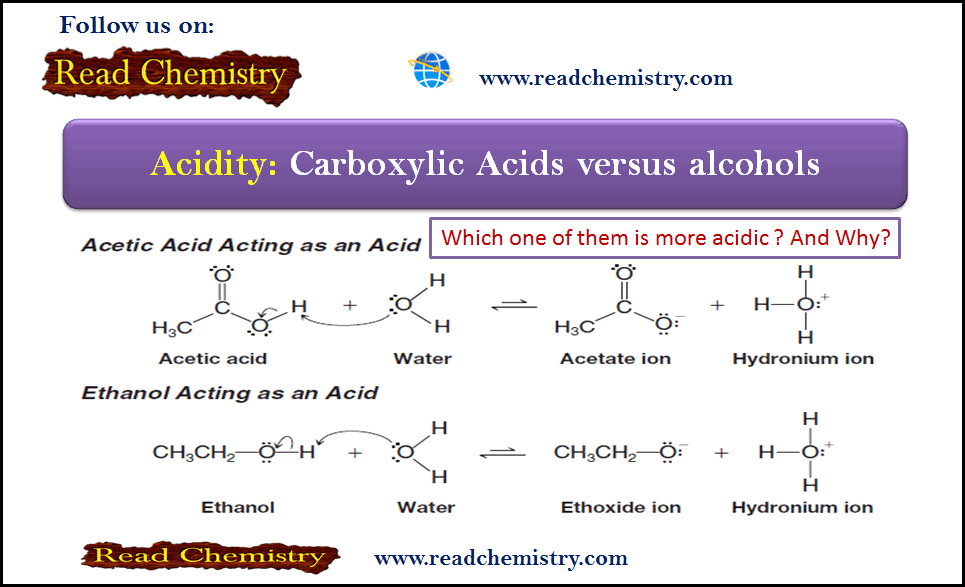
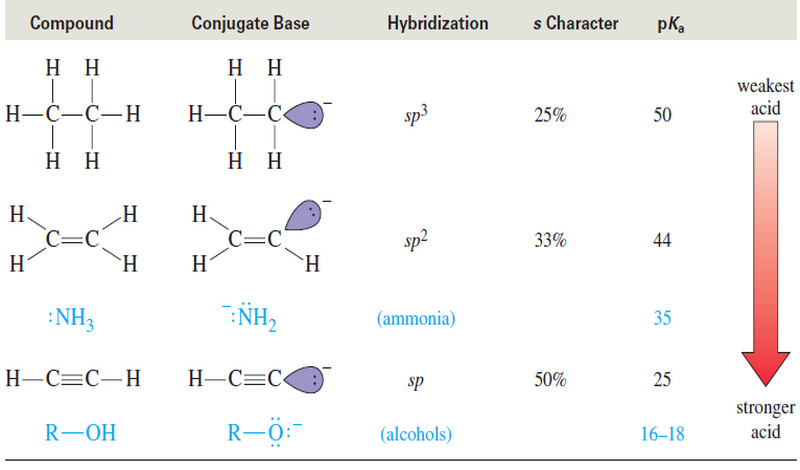
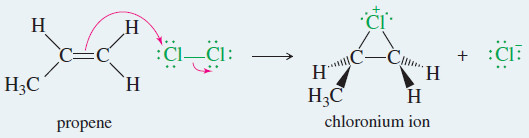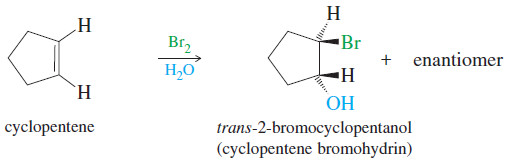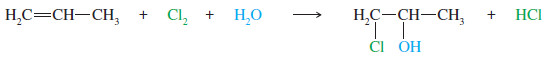Formation of Halohydrin
Formation of Halohydrin
– A halohydrin is an alcohol with a halogen on the adjacent carbon atom.
– In the presence of water, halogens add to alkenes to form halohydrins.
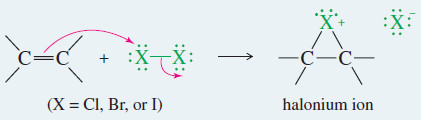
– The electrophilic halogen adds to the alkene to give a halonium ion, which is also electrophilic.
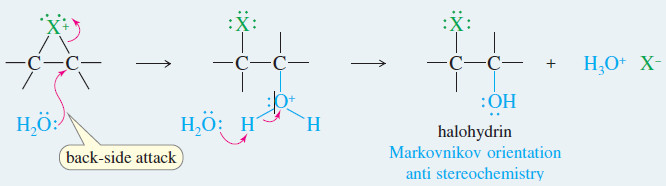
– Water acts as a nucleophile to open the halonium ion and form the halohydrin.
Mechanism: Formation of Halohydrin
Step 1: Electrophilic attack forms a halonium ion.
Step 2: Water opens the halonium ion; deprotonation gives the halohydrin.
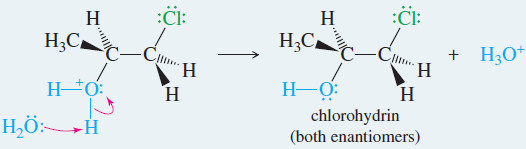
Example: Addition of Cl2 to propene in water.
Step 1: Electrophilic attack forms a chloronium ion.
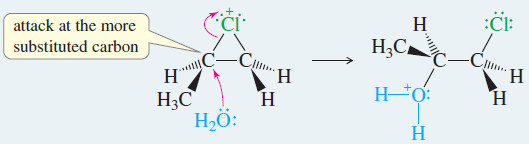
Step 2: Back-side attack by water opens the chloronium ion.
Step.3: Water removes a proton to give the chlorohydrin.
– When halogenation takes place with no solvent or with an inert solvent such as carbon tetrachloride CCl4 or chloroform CHCl3 only the halide ion is available as a nucleophile to attack the halonium ion. A dihalide results.
– But when an alkene reacts with a halogen in the presence of a nucleophilic solvent such as water, a solvent molecule is the most likely nucleophile to attack the halonium ion.
– When a water molecule attacks the halonium ion, the final product is a halohydrin, with a halogen on one carbon atom and a hydroxyl group on the adjacent carbon.
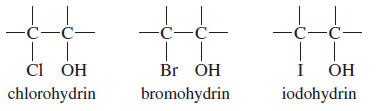
– The product may be a chlorohydrin, a bromohydrin, or an iodohydrin, depending on the halogen.
Stereochemistry of Halohydrin Formation
– Because the mechanism involves a halonium ion, the stereochemistry of addition is anti, as in halogenation.
– For example, the addition of bromine water to cyclopentene gives trans-2 bromocyclopentanol, the product of anti addition across the double bond.
Orientation of Halohydrin Formation
– Even though a halonium ion is involved, rather than a carbocation, the extended version of Markovnikov’s rule applies to halohydrin formation.
– When propene reacts with chlorine water, the major product has the electrophile (the chlorine atom) bonded to the less substituted carbon of the double bond.
– The nucleophile (the hydroxyl group) is bonded to the more substituted carbon.
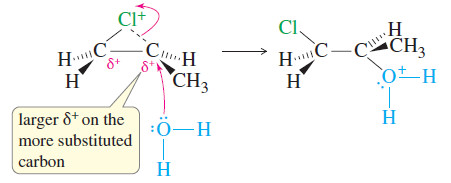
– The Markovnikov orientation observed in halohydrin formation is explained by the structure of the halonium ion intermediate.
– The two carbon atoms bonded to the halogen have partial positive charges, with a larger charge (and a weaker bond to the halogen) on the more substituted carbon atom (Figure above).
– The nucleophile (water) attacks this more substituted, more electrophilic carbon atom.
– The result is both anti stereochemistry and Markovnikov orientation.
– This halonium ion mechanism can be used to explain and predict a wide variety of reactions in both nucleophilic and non-nucleophilic solvents.
– The halonium ion mechanism is similar to the mercurinium ion mechanism for oxymercuration of an alkene, and both give Markovnikov orientation.
Solved Problem
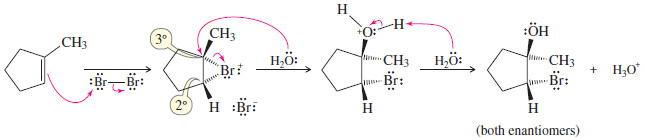
(1) Propose a mechanism for the reaction of 1-methylcyclopentene with bromine water.
Solution:
– 1-Methylcyclopentene reacts with bromine to give a bromonium ion.
– Attack by water could occur at either the secondary carbon or the tertiary carbon of the bromonium ion.
– Attack actually occurs at the more substituted carbon, which bears more of the positive charge.
– The product is formed as a racemic mixture
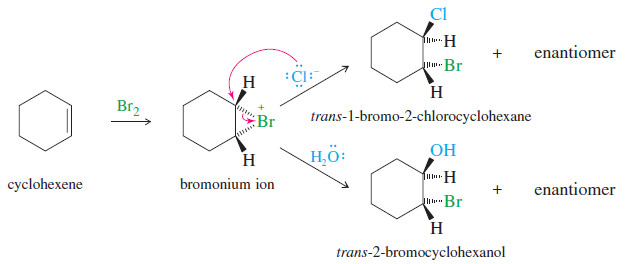
(2) When cyclohexene is treated with bromine in saturated aqueous sodium chloride, a mixture of trans-2-bromocyclohexanol and trans-1-bromo-2-chlorocyclohexane results. Propose a mechanism to account for these two products.
Solution:
– Cyclohexene reacts with bromine to give a bromonium ion, which will react with any available
nucleophile.
– The most abundant nucleophiles in saturated aqueous sodium chloride solution are water and chloride ions.
– Attack by water gives the bromohydrin, and attack by chloride gives the dihalide. Either of these attacks gives anti stereochemistry.