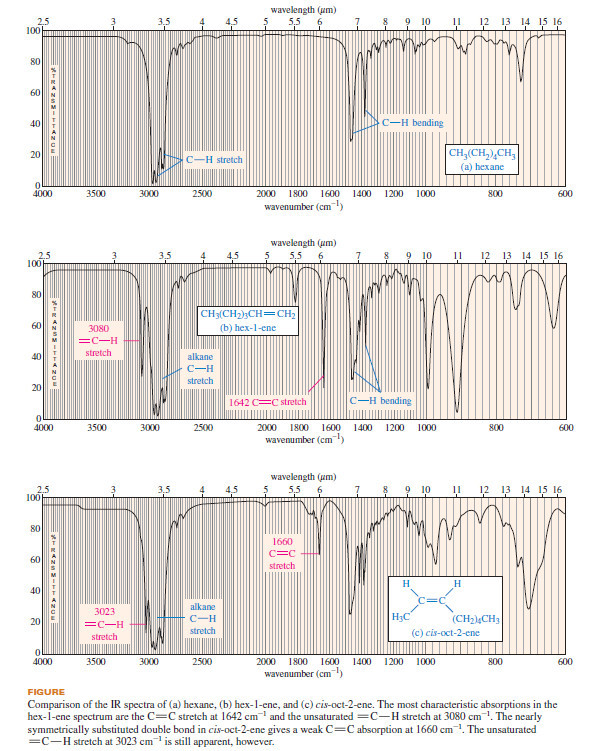
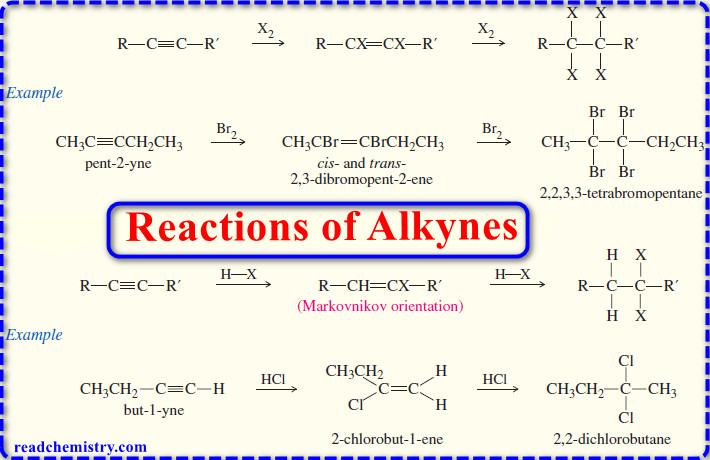
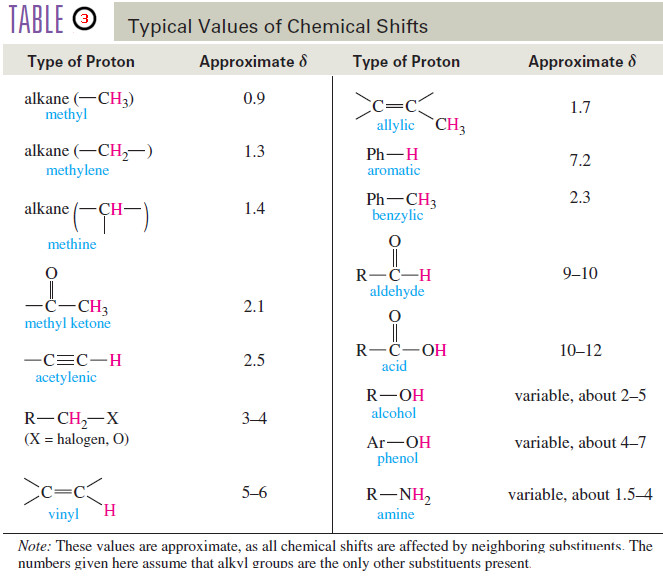
Polymerization of Alkenes
Polymerization of Alkenes
– A polymer is a large molecule composed of many smaller repeating units (the monomers) bonded together.
– Alkenes serve as monomers for some of the most common polymers, such as polyethylene, polypropylene, polystyrene, poly(vinyl chloride), and many others.
– Alkenes polymerize to give addition polymers resulting from repeated addition reactions across their double bonds.
– Addition polymers generally form by chain-growth polymerization, the rapid addition of one molecule at a time to a growing polymer chain.
– There is generally a reactive intermediate (cation, anion, or radical) at the growing end of the chain.
– Many alkenes form addition polymers under the right conditions.
– The chain-growth mechanism involves addition of the reactive end of the growing chain across the double bond of the alkene monomer.
– Depending on the conditions and the structure of the monomer, the reactive intermediates may be carbocations, free radicals, or carbanions.
(1) Cationic Polymerization
– Alkenes that easily form carbocations are good candidates for cationic polymerization, which is just another example of electrophilic addition to an alkene.
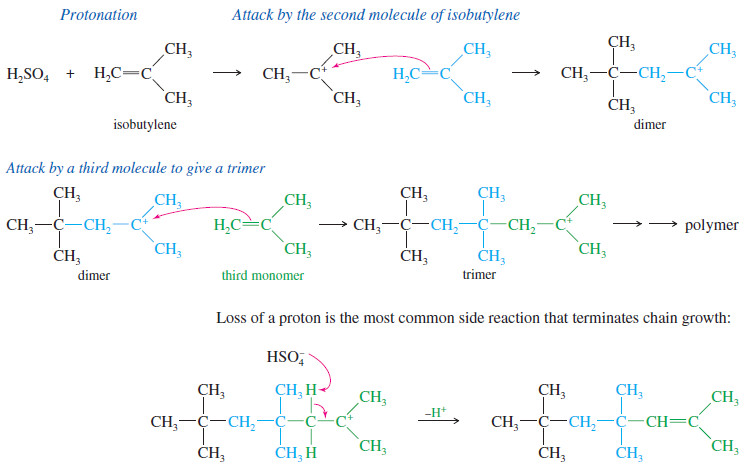
– Consider what happens when pure isobutylene is treated with a trace of concentrated sulfuric acid. Protonation of the alkene forms a carbocation.
– If a large concentration of isobutylene is available, another molecule of the alkene may act as the nucleophile and attack the carbocation to form the dimer (two monomers joined together) and give another carbocation.
– If the conditions are right, the growing cationic end of the chain will keep adding across more molecules of the monomer.
– The polymer of isobutylene is polyisobutylene, one of the constituents of butyl rubber used in inner tubes and other synthetic rubber products.
BF3 as catalyst for cationic polymerization
– Boron trifluoride (BF3) is an excellent catalyst for cationic polymerization because it leaves no good nucleophile that might attack a carbocation intermediate and end the polymerization.
– Boron trifluoride is electron-deficient and a strong Lewis acid.
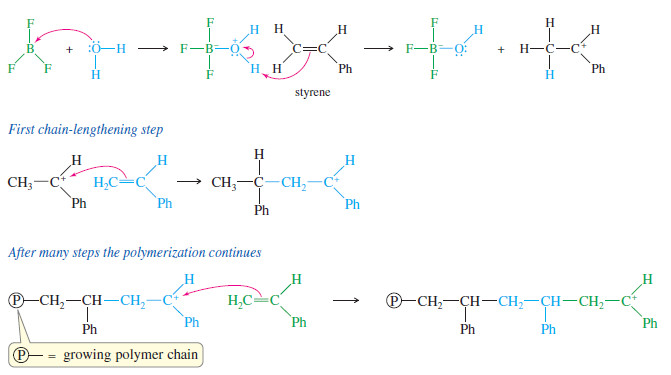
– It usually contains a trace of water that acts as a co-catalyst by adding to (BF3) and then protonating the monomer.
– Protonation occurs at the less substituted end of an alkene double bond to give the more stable carbocation.
– Each additional monomer molecule adds with the same orientation, always giving the more stable carbocation.
– The following reaction shows the polymerization of styrene (vinylbenzene) using BF3 as the catalyst
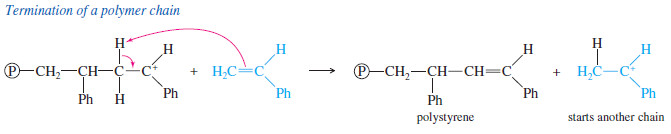
– The most likely ending of this BF3 – catalyzed polymerization is the loss of a proton from the carbocation at the end of the chain.
– This side reaction terminates one chain, but it also protonates another molecule of styrene, initiating a new chain
– The product of this polymerization is polystyrene: a clear, brittle plastic that is often used for inexpensive lenses, transparent containers, and styrofoam insulation.
– Polystyrene is also the major component of the resin beads that are used to make synthetic proteins.
(2) Free-Radical Polymerization
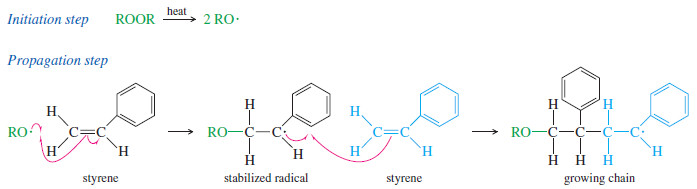
– Many alkenes undergo free-radical polymerization when they are heated with radical initiators.
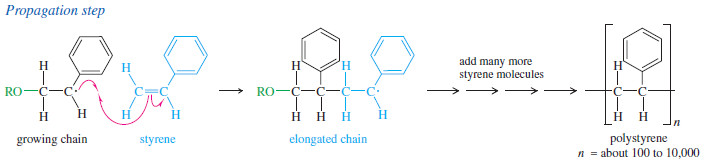
– For example, styrene polymerizes to polystyrene when it is heated to 100 Co with a peroxide initiator.
– A radical adds to styrene to give a resonance-stabilized radical, which then attacks another molecule of styrene to give an elongated radical.
– Each propagation step adds another molecule of styrene to the radical end of the growing chain.
– This addition always takes place with the orientation that gives another resonance-stabilized benzylic (next to a benzene ring) radical.
– Chain growth may continue with addition of several hundred or several thousand styrene units.
– Eventually, the chain reaction stops, either by the coupling of two chains or by reaction with an impurity (such as oxygen) or simply by running out of monomer.
– Ethylene is also polymerized by free-radical chain-growth polymerization.
– With ethylene, the free-radical intermediates are less stable, so stronger reaction conditions are required.
– Ethylene is commonly polymerized by free-radical initiators at pressures around 3000 atm and temperatures of about 200 oC. The product, called low-density polyethylene, is the material commonly used in polyethylene bags.
(3) Anionic Polymerization
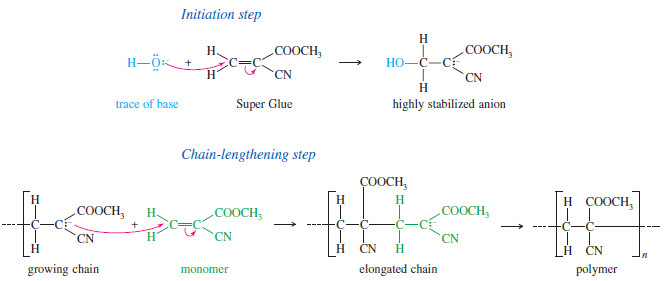
– Like cationic polymerization, anionic polymerization depends on the presence of a stabilizing group.
– To stabilize anions, the double bond should have a strong electronwithdrawing group such as a carbonyl group, a cyano group, or a nitro group.
– Methyl α-cyanoacrylate contains two powerful electron-withdrawing groups, and it undergoes nucleophilic additions very easily.
– If this liquid monomer is spread in a thin film between two surfaces, traces of basic impurities (metal oxides, etc.) can catalyze its rapid polymerization.
– The solidified polymer joins the two surfaces.
– The chemists who first made this monomer noticed how easily it polymerizes and realized that it could serve as a fast-setting glue.
– Methyl α-cyanoacrylate is sold commercially as Super Glue.