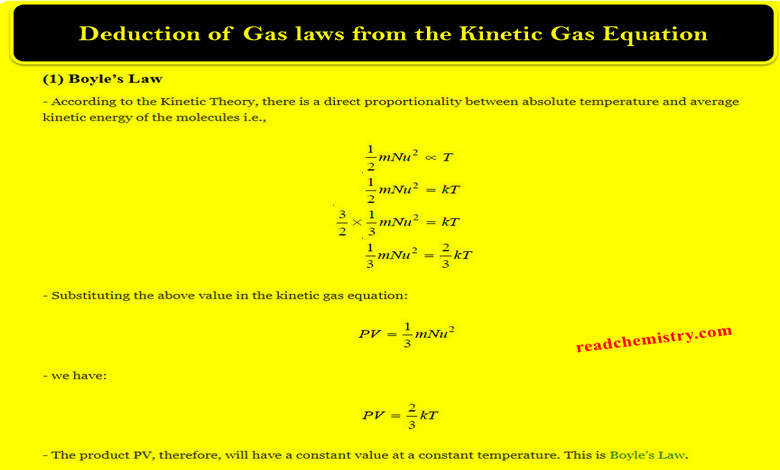
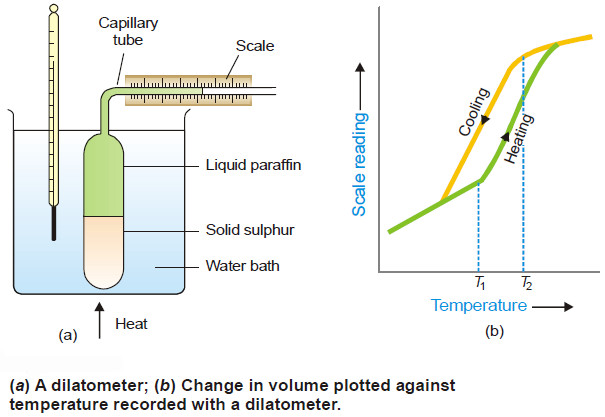
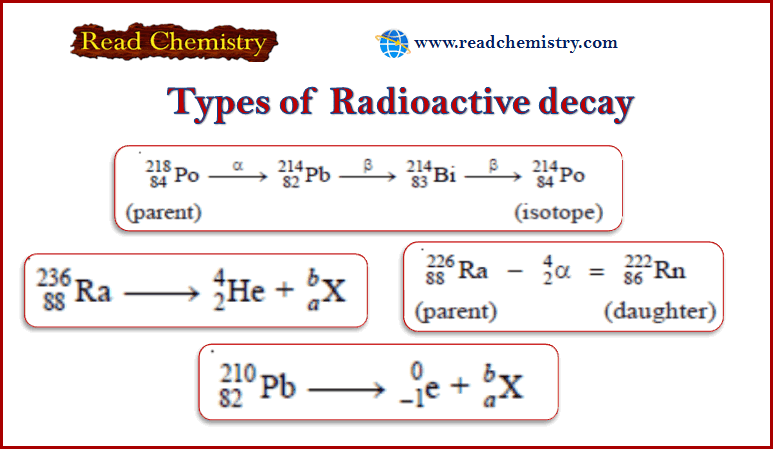
Degree of Freedom – phase Rule
Degree of Freedom
– The term Degree of Freedom represented by F in the phase Rule equation (F = C – P + 2) is defined as follows :
the least number of variable factors (concentration, pressure and temperature) which must be specified so that the remaining variables are fixed automatically and the system is completely defined
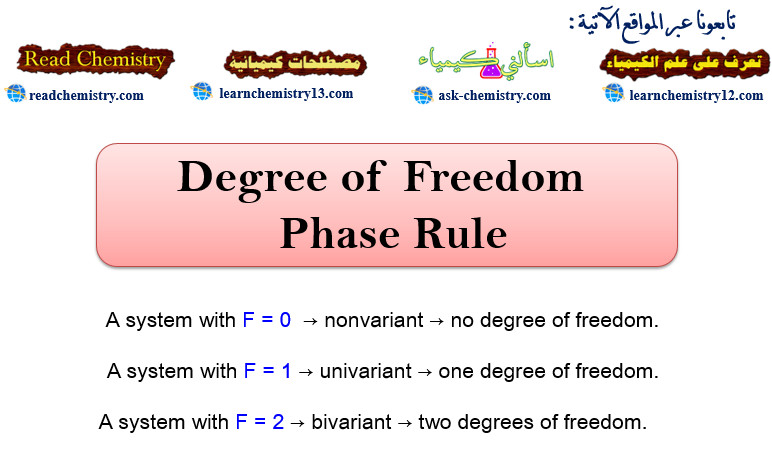
– A system with F = 0 is known as nonvariant or having no degree of freedom.
– but A system with F = 1 is known as univariant or having one degree of freedom.
– A system with F = 2 is known as bivariant or having two degrees of freedom.
Explanation of the Term Degree of Freedom
– A system is defined completely when it retains the same state of equilibrium (or can be reproduced exactly) with the specified variables.
– Let us consider some examples:-
(1) For a pure gas, F = 2
– For a given sample of any pure gas PV = RT.
– If the values of pressure (P) and temperature (T) be specified, volume (V) can have only one definite value, or that the volume (the third variable) is fixed automatically.
– Any other sample of the gas under the same pressure and temperature as specified above, will be identical with the first one.
– Hence a system containing a pure gas has two degrees of freedom (F = 2).
(2) For a mixture of gases, F = 3
– A system containing a mixture of two or more gases is completely defined when its composition, temperature and pressure are specified.
– If pressure and temperature only are specified, the third variable i.e., composition could be varied.
– Since it is necessary to specify three variables to define the system completely, a mixture of gases has three degrees of freedom (F = 3).
(3) For water ↔ water vapour, F = 1
– The system water in equilibrium with water vapour, has two variables temperature and pressure.
– At a definite temperature the vapour pressure of water can have only one fixed value.
– Thus if one variable (temperature or pressure) is specified, the other is fixed automatically.
– Hence the system water has one degree of freedom (F = 1).
(4) For saturated NaCl solution, F = 1
– The saturated solution of sodium chloride in equilibrium with solid sodium chloride and water vapour.
NaCl (solid) ↔ NaCl-solution ↔ water vapour
– Thus the system is completely defined if we specify temperature only.
– The other two variables i.e,. the composition of NaCl-solution (solubility) and vapour pressure have a definite value at a fixed temperature.
– Hence the system has one degree of freedom.
(5) For ice-water-vapour system, F = 0
– In the system ice ↔ water ↔ vapour, the three phases coexist at the freezing point of water.
– Since the freezing temperature of water has a fixed value, the vapour pressure of water has also a definite value.
– The system has two variables (temperature and pressure) and both these are already fixed.
– Thus the system is completely defined automatically, there being no need to specify any variable.
– Hence it has no degree of freedom (F = 0).